Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T86591
(Former ID: TTDS00340)
|
|||||
| Target Name |
Peroxisome proliferator-activated receptor alpha (PPARA)
|
|||||
| Synonyms |
Peroxisome proliferater-activated receptor alpha; PPARalpha; PPAR-alpha; PPAR; Nuclear receptor subfamily 1 group C member 1; NR1C1
Click to Show/Hide
|
|||||
| Gene Name |
PPARA
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Hyper-lipoproteinaemia [ICD-11: 5C80] | |||||
| 2 | Hyperlipidemia [ICD-11: 5C80] | |||||
| 3 | Type 2 diabetes mellitus [ICD-11: 5A11] | |||||
| Function |
Key regulator of lipid metabolism. Activated by the endogenous ligand 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC). Activated by oleylethanolamide, a naturally occurring lipid that regulates satiety. Receptor for peroxisome proliferators such as hypolipidemic drugs and fatty acids. Regulates the peroxisomal beta-oxidation pathway of fatty acids. Functions as transcription activator for the ACOX1 and P450 genes. Transactivation activity requires heterodimerization with RXRA and is antagonized by NR2C2. May be required for the propagation of clock information to metabolic pathways regulated by PER2. Ligand-activated transcription factor.
Click to Show/Hide
|
|||||
| BioChemical Class |
Nuclear hormone receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSC
PGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACE GCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSE KAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFV IHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANL DLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFD FAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDI FLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T49ORJ | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 5 Approved Drugs | + | ||||
| 1 | Bezafibrate | Drug Info | Approved | Hyperlipidaemia | [2], [3] | |
| 2 | Ciprofibrate | Drug Info | Approved | Hyperlipoproteinemia | [3], [4], [5] | |
| 3 | Fenofibrate | Drug Info | Approved | High cholesterol level | [6], [7] | |
| 4 | Lobeglitazone | Drug Info | Approved | Type-2 diabetes | [8] | |
| 5 | Pemafibrate | Drug Info | Approved | Hyperlipidemia | [9] | |
| Clinical Trial Drug(s) | [+] 16 Clinical Trial Drugs | + | ||||
| 1 | CS-038 | Drug Info | Phase 3 | Type-2 diabetes | [10] | |
| 2 | GFT-505 | Drug Info | Phase 3 | Non-alcoholic steatohepatitis | [11] | |
| 3 | Imiglitazar | Drug Info | Phase 3 | Type-2 diabetes | [12], [13] | |
| 4 | Ragaglitazar | Drug Info | Phase 3 | Type-1 diabetes | [14], [15] | |
| 5 | TESAGLITAZAR | Drug Info | Phase 3 | Type-1 diabetes | [16] | |
| 6 | ZYH-1 | Drug Info | Phase 3 | Lipid metabolism disorder | [17] | |
| 7 | GFT14 | Drug Info | Phase 2 | Hyperlipidaemia | [18] | |
| 8 | LY-518674 | Drug Info | Phase 2 | Diabetic complication | [19], [20] | |
| 9 | Naveglitazar | Drug Info | Phase 2 | Diabetic complication | [21] | |
| 10 | ONO-5129 | Drug Info | Phase 2 | Diabetic complication | [22] | |
| 11 | ZYH7 | Drug Info | Phase 2 | Lipid metabolism disorder | [23] | |
| 12 | AVE0897 | Drug Info | Phase 1 | Type-2 diabetes | [24] | |
| 13 | CDT-fenofibrate | Drug Info | Phase 1 | Hyperlipidaemia | [25] | |
| 14 | GW-409544 | Drug Info | Phase 1 | Hyperlipidaemia | [26], [27] | |
| 15 | Oxeglitazar | Drug Info | Phase 1 | Gout | [28] | |
| 16 | TPST-1120 | Drug Info | Phase 1 | Solid tumour/cancer | [29] | |
| Discontinued Drug(s) | [+] 22 Discontinued Drugs | + | ||||
| 1 | Aleglitazar | Drug Info | Discontinued in Phase 3 | Type-2 diabetes | [30], [31] | |
| 2 | AVE-0847 | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [32] | |
| 3 | AVE-8134 | Drug Info | Discontinued in Phase 2 | Heart failure | [33] | |
| 4 | BM-17.0744 | Drug Info | Discontinued in Phase 2 | Type-1 diabetes | [34] | |
| 5 | GSK-677954 | Drug Info | Discontinued in Phase 2 | Non-alcoholic fatty liver disease | [35] | |
| 6 | Indeglitazar | Drug Info | Discontinued in Phase 2 | Type-2 diabetes | [36] | |
| 7 | KRP-101 | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [37] | |
| 8 | KRP-297 | Drug Info | Discontinued in Phase 2 | Type-2 diabetes | [38] | |
| 9 | NS-220 | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [39], [40] | |
| 10 | Reglixane | Drug Info | Discontinued in Phase 2 | Diabetic complication | [41] | |
| 11 | Sodelglitazar | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [42] | |
| 12 | AR-H049020 | Drug Info | Discontinued in Phase 1 | Type-1 diabetes | [43] | |
| 13 | DRF 10945 | Drug Info | Discontinued in Phase 1 | Hyperlipidaemia | [44] | |
| 14 | E-3030 | Drug Info | Discontinued in Phase 1 | Hyperlipidaemia | [45] | |
| 15 | LG-101280 | Drug Info | Discontinued in Phase 1 | Arteriosclerosis | [46] | |
| 16 | LY-929 | Drug Info | Discontinued in Phase 1 | Lipid metabolism disorder | [47], [46] | |
| 17 | MP-136 | Drug Info | Discontinued in Phase 1 | Lipid metabolism disorder | [48] | |
| 18 | BVT-142 | Drug Info | Terminated | Type-2 diabetes | [53] | |
| 19 | CS-204 | Drug Info | Terminated | Metabolic disorder | [54] | |
| 20 | CS-207 | Drug Info | Terminated | Cardiovascular disease | [55] | |
| 21 | KRP-105 | Drug Info | Terminated | Lipid metabolism disorder | [56] | |
| 22 | Sipoglitazar | Drug Info | Terminated | Diabetic complication | [57] | |
| Preclinical Drug(s) | [+] 4 Preclinical Drugs | + | ||||
| 1 | MC-3001 | Drug Info | Preclinical | Lipid metabolism disorder | [49] | |
| 2 | MC-3002 | Drug Info | Preclinical | Metabolic disorder | [50] | |
| 3 | PIRINIXIC ACID | Drug Info | Preclinical | Pulmonary fibrosis | [51] | |
| 4 | Romazarit | Drug Info | Preclinical | Rheumatoid arthritis | [52] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Agonist | [+] 43 Agonist drugs | + | ||||
| 1 | Bezafibrate | Drug Info | [58] | |||
| 2 | Ciprofibrate | Drug Info | [1] | |||
| 3 | Fenofibrate | Drug Info | [1] | |||
| 4 | Lobeglitazone | Drug Info | [8] | |||
| 5 | Pemafibrate | Drug Info | [9] | |||
| 6 | GFT14 | Drug Info | [66] | |||
| 7 | LY-518674 | Drug Info | [67] | |||
| 8 | ZYH7 | Drug Info | [70] | |||
| 9 | AVE0897 | Drug Info | [71] | |||
| 10 | CDT-fenofibrate | Drug Info | [25] | |||
| 11 | Flavonoid derivative 8 | Drug Info | [73] | |||
| 12 | PMID25416646-Compound-Figure5-A | Drug Info | [73] | |||
| 13 | PMID25416646-Compound-Figure5-H | Drug Info | [73] | |||
| 14 | Aleglitazar | Drug Info | [74] | |||
| 15 | AVE-8134 | Drug Info | [76] | |||
| 16 | GSK-677954 | Drug Info | [35] | |||
| 17 | Indeglitazar | Drug Info | [78] | |||
| 18 | KRP-101 | Drug Info | [79], [80] | |||
| 19 | NS-220 | Drug Info | [5], [82] | |||
| 20 | Sodelglitazar | Drug Info | [83] | |||
| 21 | AR-H049020 | Drug Info | [84] | |||
| 22 | DRF 10945 | Drug Info | [85] | |||
| 23 | LY-929 | Drug Info | [88] | |||
| 24 | MP-136 | Drug Info | [89] | |||
| 25 | MC-3001 | Drug Info | [90] | |||
| 26 | BVT-142 | Drug Info | [53], [93] | |||
| 27 | CS-204 | Drug Info | [94] | |||
| 28 | CS-207 | Drug Info | [95] | |||
| 29 | KRP-105 | Drug Info | [96] | |||
| 30 | (E)-4-(3,5-dimethoxystyryl)phenol | Drug Info | [89] | |||
| 31 | 8S-HETE | Drug Info | [98] | |||
| 32 | AD-5061 | Drug Info | [62] | |||
| 33 | CP-775146 | Drug Info | [100] | |||
| 34 | DB-900 | Drug Info | [101] | |||
| 35 | DRF 2519 | Drug Info | [103] | |||
| 36 | eicosatetranoic acid | Drug Info | [104] | |||
| 37 | Fibrates | Drug Info | [7] | |||
| 38 | GW7647 | Drug Info | [107] | |||
| 39 | LY-465608 | Drug Info | [109] | |||
| 40 | N-oleoylethanolamide | Drug Info | [110] | |||
| 41 | pristanic acid | Drug Info | [111] | |||
| 42 | reglitazar | Drug Info | [112] | |||
| 43 | TZD18 | Drug Info | [113] | |||
| Modulator | [+] 22 Modulator drugs | + | ||||
| 1 | CS-038 | Drug Info | [59], [60] | |||
| 2 | GFT-505 | Drug Info | [61] | |||
| 3 | Imiglitazar | Drug Info | [62], [63] | |||
| 4 | MURAGLITAZAR | Drug Info | [64] | |||
| 5 | Ragaglitazar | Drug Info | [15] | |||
| 6 | TESAGLITAZAR | Drug Info | [65] | |||
| 7 | ZYH-1 | Drug Info | [57] | |||
| 8 | Naveglitazar | Drug Info | [68] | |||
| 9 | ONO-5129 | Drug Info | [69] | |||
| 10 | GW-409544 | Drug Info | [27] | |||
| 11 | Oxeglitazar | Drug Info | [28] | |||
| 12 | AVE-0847 | Drug Info | [75] | |||
| 13 | KRP-297 | Drug Info | [81] | |||
| 14 | Reglixane | Drug Info | [69] | |||
| 15 | E-3030 | Drug Info | [86] | |||
| 16 | LG-101280 | Drug Info | [87] | |||
| 17 | MC-3002 | Drug Info | [69] | |||
| 18 | Romazarit | Drug Info | [92] | |||
| 19 | Sipoglitazar | Drug Info | [57] | |||
| 20 | GW-2331 | Drug Info | [106] | |||
| 21 | LL-6531 | Drug Info | [89] | |||
| 22 | ZY H2 | Drug Info | [114] | |||
| Antagonist | [+] 1 Antagonist drugs | + | ||||
| 1 | TPST-1120 | Drug Info | [72] | |||
| Activator | [+] 1 Activator drugs | + | ||||
| 1 | BM-17.0744 | Drug Info | [5], [77] | |||
| Inhibitor | [+] 9 Inhibitor drugs | + | ||||
| 1 | PIRINIXIC ACID | Drug Info | [91] | |||
| 2 | (9Z,12E)-12-nitrooctadeca-9,12-dienoic acid | Drug Info | [97] | |||
| 3 | (E)-12-Nitrooctadec-12-enoic Acid | Drug Info | [97] | |||
| 4 | (E)-13-Nitrooctadec-12-enoic Acid | Drug Info | [97] | |||
| 5 | BMS-687453 | Drug Info | [99] | |||
| 6 | Deoxy-Bigchap | Drug Info | [102] | |||
| 7 | GSK-9578 | Drug Info | [1], [105] | |||
| 8 | L-165461 | Drug Info | [108] | |||
| 9 | L-796449 | Drug Info | [108] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Icosapent | Ligand Info | |||||
| Structure Description | X-ray structure of human PPARalpha ligand binding domain-eicosapentaenoic acid (EPA) co-crystals obtained by delipidation and cross-seeding | PDB:6LXA | ||||
| Method | X-ray diffraction | Resolution | 1.23 Å | Mutation | No | [115] |
| PDB Sequence |
MTADLKSLAK
208 RIYEAYLKNF218 NMNKVKARVI228 LSGKSNNPPF239 VIHDMETLCM249 AEKTLVAKLV 259 ANGIQNKEAE269 VRIFHCCQCT279 SVETVTELTE289 FAKAIPGFAN299 LDLNDQVTLL 309 KYGVYEAIFA319 MLSSVMNKDG329 MLVAYGNGFI339 TREFLKSLRK349 PFCDIMEPKF 359 DFAMKFNALE369 LDDSDISLFV379 AAIICCGDRP389 GLLNVGHIEK399 MQEGIVHVLR 409 LHLQSNHPDD419 IFLFPKLLQK429 MADLRQLVTE439 HAQLVQIIKK449 TESDAALHPL 459 LQEIYRDMY
|
|||||
|
|
ILE241
3.245
LEU247
3.678
ALA250
3.165
GLU251
2.919
LEU254
3.528
VAL255
3.359
ARG271
4.586
ILE272
2.730
PHE273
2.914
CYS275
3.365
CYS276
2.942
GLN277
3.081
THR279
2.613
SER280
2.693
TYR314
2.629
ILE317
4.902
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: Pemafibrate | Ligand Info | |||||
| Structure Description | X-ray structure of human PPARalpha ligand binding domain-pemafibrate co-crystals obtained by delipidation and cross-seeding | PDB:6KB4 | ||||
| Method | X-ray diffraction | Resolution | 1.42 Å | Mutation | No | [115] |
| PDB Sequence |
MTADLKSLAK
208 RIYEAYLKNF218 NMNKVKARVI228 LSGSNNPPFV240 IHDMETLCMA250 EKTLVAKLVA 260 NGIQNKEAEV270 RIFHCCQCTS280 VETVTELTEF290 AKAIPGFANL300 DLNDQVTLLK 310 YGVYEAIFAM320 LSSVMNKDGM330 LVAYGNGFIT340 REFLKSLRKP350 FCDIMEPKFD 360 FAMKFNALEL370 DDSDISLFVA380 AIICCGDRPG390 LLNVGHIEKM400 QEGIVHVLRL 410 HLQSNHPDDI420 FLFPKLLQKM430 ADLRQLVTEH440 AQLVQIIKKT450 ESDAALHPLL 460 QEIYRDMY
|
|||||
|
|
MET220
3.259
ILE241
2.791
LEU247
3.962
ALA250
3.874
GLU251
3.640
LEU254
4.853
VAL255
4.161
ILE272
3.791
PHE273
2.680
CYS275
3.619
CYS276
2.687
GLN277
3.223
THR279
2.889
SER280
2.569
THR283
3.993
TYR314
2.736
ALA316
4.941
ILE317
3.151
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
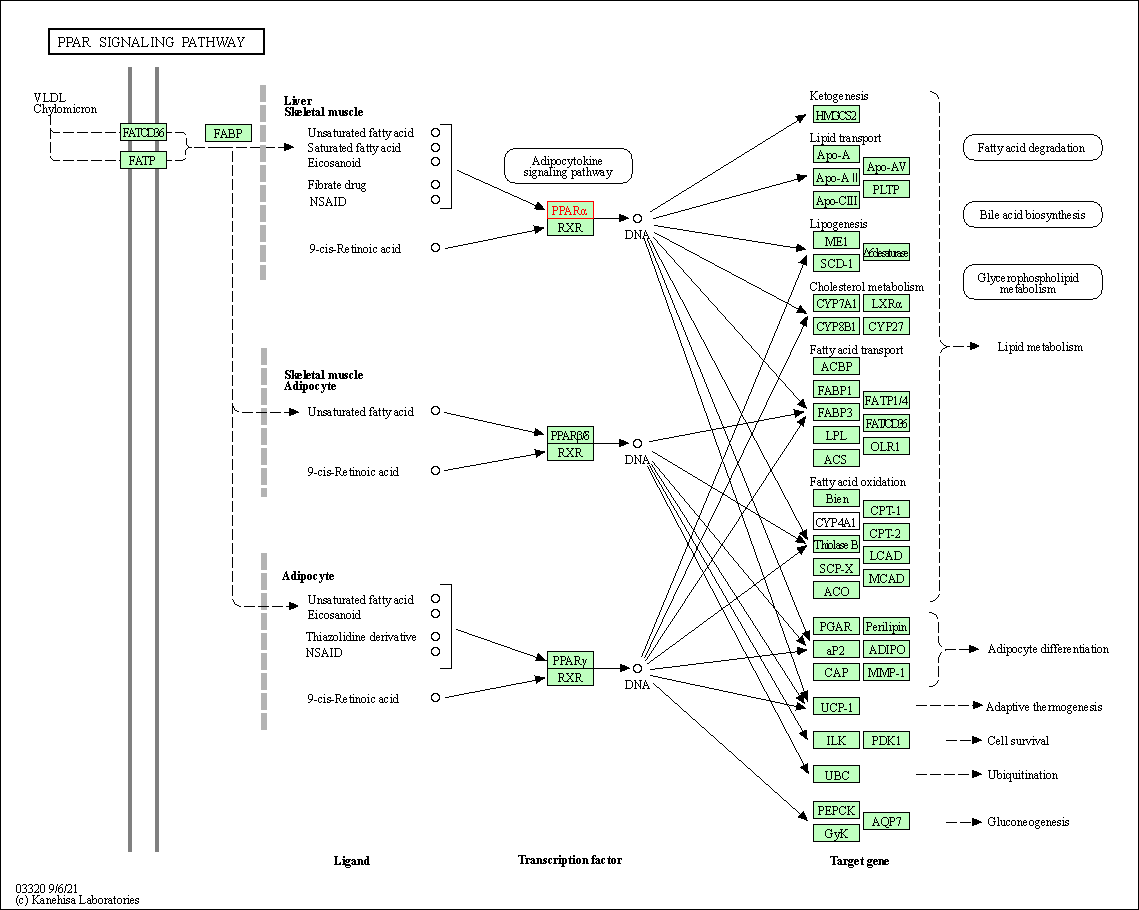
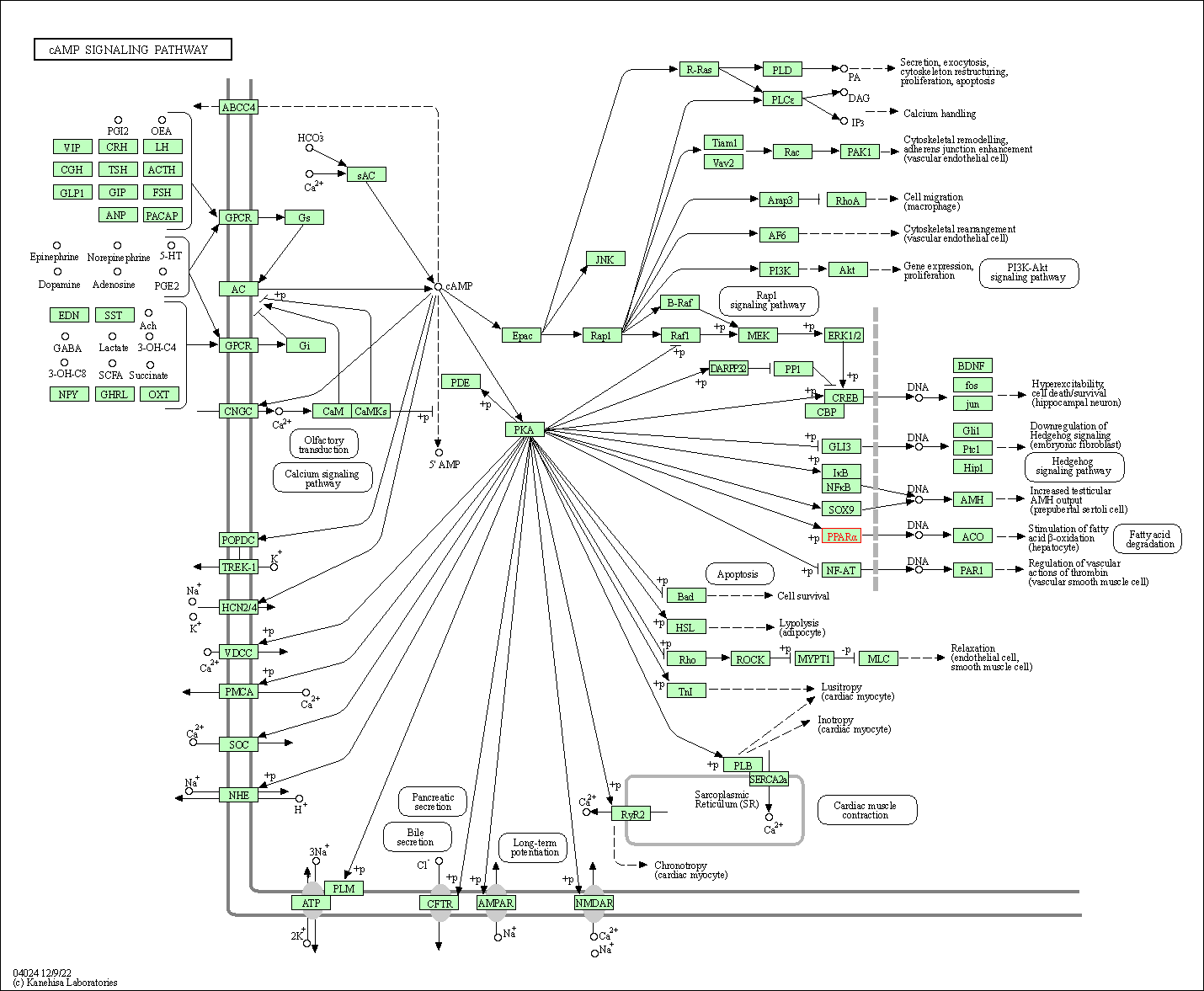
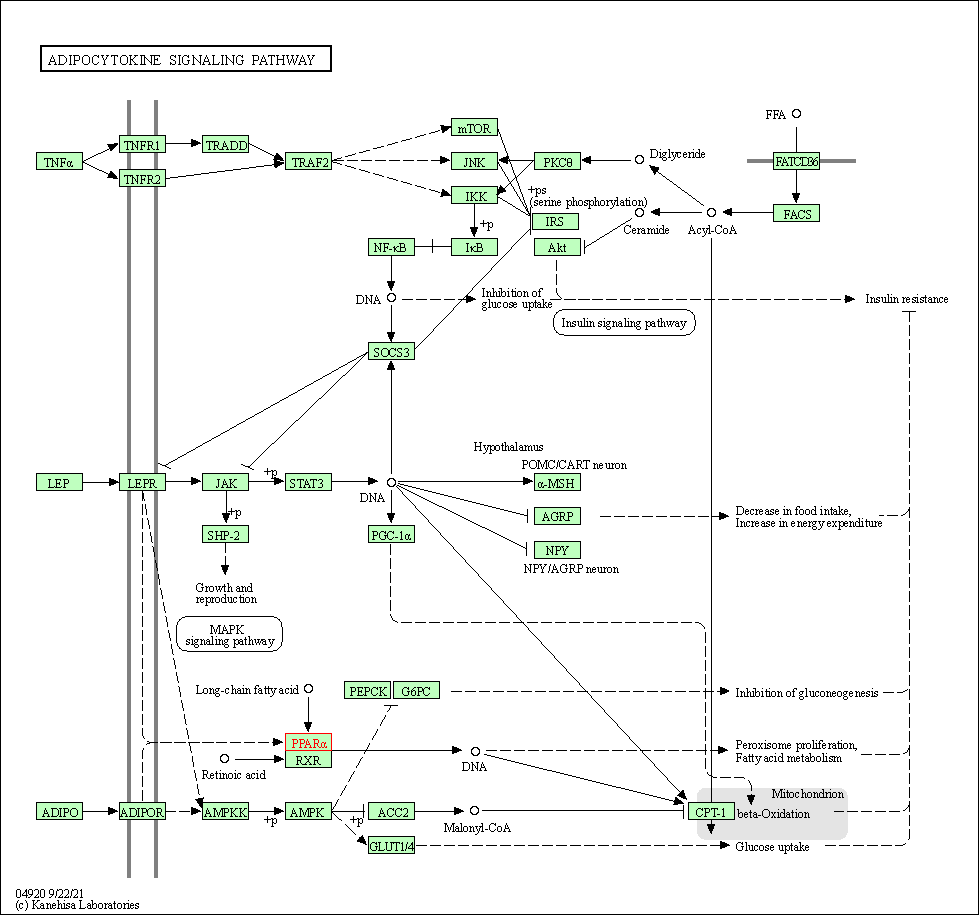
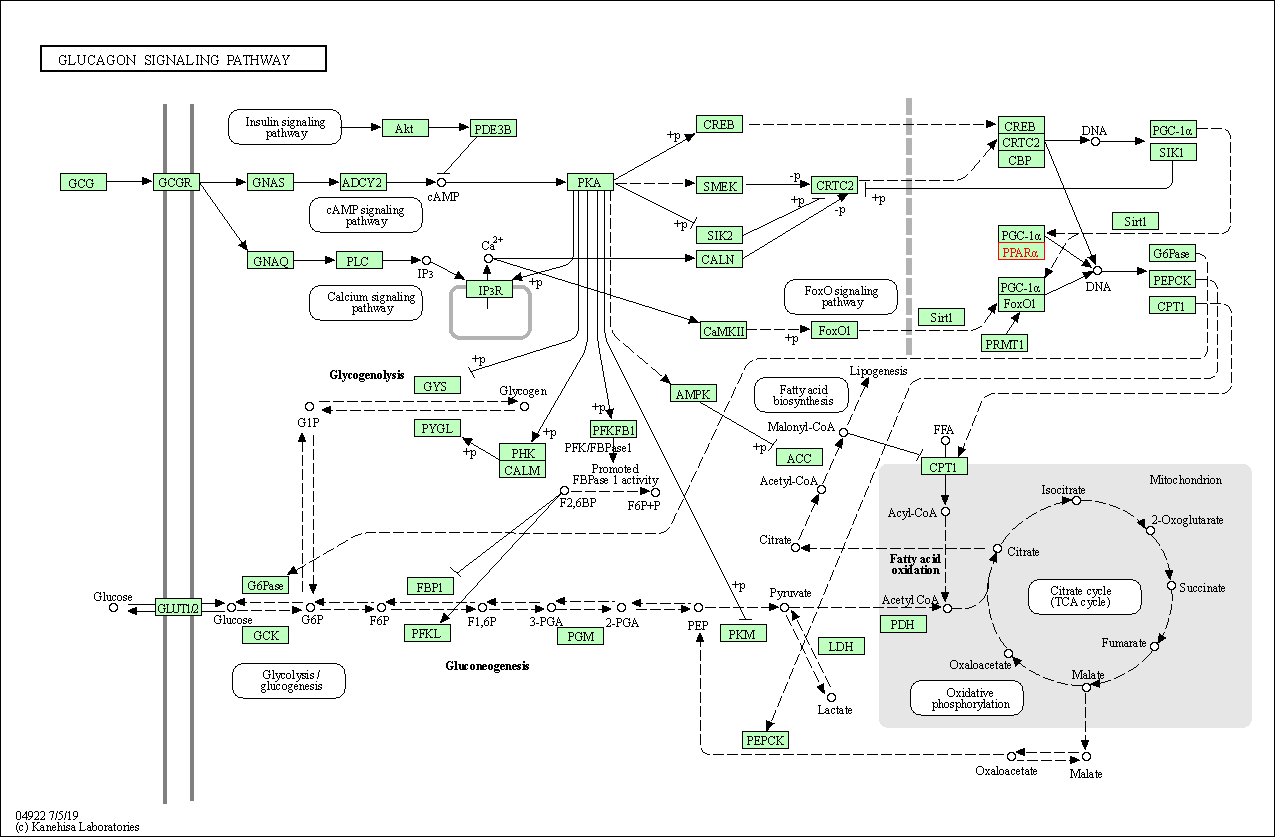
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| PPAR signaling pathway | hsa03320 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| cAMP signaling pathway | hsa04024 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Adipocytokine signaling pathway | hsa04920 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Glucagon signaling pathway | hsa04922 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 49 | Degree centrality | 5.26E-03 | Betweenness centrality | 2.28E-02 |
|---|---|---|---|---|---|
| Closeness centrality | 2.57E-01 | Radiality | 1.44E+01 | Clustering coefficient | 8.76E-02 |
| Neighborhood connectivity | 3.16E+01 | Topological coefficient | 3.93E-02 | Eccentricity | 10 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000 Jun 2;275(22):16638-42. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2668). | |||||
| REF 3 | Antidiabetic action of bezafibrate in a large observational database. Diabetes Care. 2009 Apr;32(4):547-51. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3438). | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7186). | |||||
| REF 7 | Emerging antidyslipidemic drugs. Expert Opin Emerg Drugs. 2008 Jun;13(2):363-81. | |||||
| REF 8 | Tolerability and pharmacokinetics of lobeglitazone, a novel peroxisome proliferator-activated receptor-gamma agonist, after a single oral administration in healthy female subjects. Clin Drug Investig. 2014 Jul;34(7):467-74. | |||||
| REF 9 | Pemafibrate: First Global Approval. Drugs. 2017 Oct;77(16):1805-1810. | |||||
| REF 10 | ClinicalTrials.gov (NCT02173457) Study of Chiglitazar Compare With Sitagliptin in Type 2 Diabetes Patients. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT02704403) Phase 3 Study to Evaluate the Efficacy and Safety of Elafibranor Versus Placebo in Patients With Nonalcoholic Steatohepatitis (NASH) (RESOLVE-IT). U.S. National Institutes of Health. | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2675). | |||||
| REF 13 | Medicinal Chemistry and Actions of Dual and Pan PPAR Modulators. Open Med Chem J. 2011;5(Suppl 2):93-8. | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2664). | |||||
| REF 15 | Ragaglitazar: the pharmacokinetics, pharmacodynamics, and tolerability of a novel dual PPAR alpha and gamma agonist in healthy subjects and patients with type 2 diabetes. J Clin Pharmacol. 2003 Nov;43(11):1244-56. | |||||
| REF 16 | ClinicalTrials.gov (NCT00229710) GALLEX 9: Safety and Tolerability of Oral Tesaglitazar When Added to Insulin Therapy in Patients With Type 2 Diabetes. U.S. National Institutes of Health. | |||||
| REF 17 | A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 g compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI). Diabetes Technol Ther. 2014 Feb;16(2):63-71. | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024296) | |||||
| REF 19 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2658). | |||||
| REF 20 | ClinicalTrials.gov (NCT00327002) A Mechanistic Study of the Effects of LY518674 on High-Density Lipoprotein Cholesterol (HDL-C) Metabolism. U.S. National Institutes of Health. | |||||
| REF 21 | ClinicalTrials.gov (NCT00065312) An Evaluation of an Oral Antidiabetic Agent for the Treatment of Type 2 Diabetes. U.S. National Institutes of Health. | |||||
| REF 22 | ClinicalTrials.gov (NCT00335712) Pilot Study of ONO-5129 in Patients With Type 2 Diabetes Mellitus. U.S. National Institutes of Health. | |||||
| REF 23 | ClinicalTrials.gov (NCT01539616) A Clinical Trial to Evaluate the Safety and Efficacy of ZYH7 Compared to Fenofibrate in Patients With Dyslipidemia. U.S. National Institutes of Health. | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022196) | |||||
| REF 25 | Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998 Nov 10;98(19):2088-93. | |||||
| REF 26 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3440). | |||||
| REF 27 | Design novel dual agonists for treating type-2 diabetes by targeting peroxisome proliferator-activated receptors with core hopping approach. PLoS One. 2012;7(6):e38546. | |||||
| REF 28 | Therapeutic potential of aleglitazar, a new dual PPAR-alpha/gamma agonist: implications for cardiovascular disease in patients with diabetes mellitus. Am J Cardiovasc Drugs. 2010;10(4):209-16. | |||||
| REF 29 | ClinicalTrials.gov (NCT03829436) TPST-1120 as Monotherapy and in Combination With Nivolumab in Subjects With Advanced Cancers. U.S. National Institutes of Health. | |||||
| REF 30 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7405). | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017317) | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020608) | |||||
| REF 33 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021122) | |||||
| REF 34 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011739) | |||||
| REF 35 | Emerging drugs for non-alcoholic fatty liver disease. Expert Opin Emerg Drugs. 2008 Mar;13(1):145-58. | |||||
| REF 36 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022483) | |||||
| REF 37 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018628) | |||||
| REF 38 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010490) | |||||
| REF 39 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2678). | |||||
| REF 40 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017798) | |||||
| REF 41 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008953) | |||||
| REF 42 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018093) | |||||
| REF 43 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800016001) | |||||
| REF 44 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020522) | |||||
| REF 45 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021476) | |||||
| REF 46 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800014823) | |||||
| REF 47 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2657). | |||||
| REF 48 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031057) | |||||
| REF 49 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021175) | |||||
| REF 50 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021065) | |||||
| REF 51 | Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020 Jan;19(1):57-75. | |||||
| REF 52 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000969) | |||||
| REF 53 | A new class of peroxisome proliferator-activated receptor agonists with a novel binding epitope shows antidiabetic effects. J Biol Chem. 2004 Sep 24;279(39):41124-30. | |||||
| REF 54 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026847) | |||||
| REF 55 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026848) | |||||
| REF 56 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026674) | |||||
| REF 57 | Pharmacokinetics, safety, and tolerability of saroglitazar (ZYH1), a predominantly PPARalpha agonist with moderate PPARgamma agonist activity in healthy human subjects. Clin Drug Investig. 2013 Nov;33(11):809-16. | |||||
| REF 58 | Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in... Mol Pharmacol. 2009 Apr;75(4):782-92. | |||||
| REF 59 | Determination of chiglitazar, a dual alpha/gamma peroxisome proliferator-activated receptor (PPAR) agonist, in human plasma by liquid chromatograph... Pharmazie. 2007 Nov;62(11):825-9. | |||||
| REF 60 | The PPARalpha/gamma dual agonist chiglitazar improves insulin resistance and dyslipidemia in MSG obese rats.Br J Pharmacol.2006 Jul;148(5):610-8. | |||||
| REF 61 | Dual peroxisome proliferator-activated receptor / agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects.Diabetes Care.2013 Oct;36(10):2923-30. | |||||
| REF 62 | A novel oxyiminoalkanoic acid derivative, TAK-559, activates human peroxisome proliferator-activated receptor subtypes. Eur J Pharmacol. 2004 Jul 8;495(1):17-26. | |||||
| REF 63 | A potent activator of PPARalpha and gamma reduces the vascular cell recruitment and inhibits the intimal thickning in hypercholesterolemic rabbits. Atherosclerosis. 2005 Jan;178(1):1-7. | |||||
| REF 64 | Muraglitazar, a dual (alpha/gamma) PPAR activator: a randomized, double-blind, placebo-controlled, 24-week monotherapy trial in adult patients with... Clin Ther. 2005 Aug;27(8):1181-95. | |||||
| REF 65 | Tesaglitazar, a dual PPAR-/ agonist, hamster carcinogenicity, investigative animal and clinical studies.Toxicol Pathol.2012;40(1):18-32. | |||||
| REF 66 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024296) | |||||
| REF 67 | Potent and selective PPAR-alpha agonist LY518674 upregulates both ApoA-I production and catabolism in human subjects with the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2009 Jan;29(1):140-6. | |||||
| REF 68 | The disposition and metabolism of naveglitazar, a peroxisome proliferator-activated receptor alpha-gamma dual, gamma-dominant agonist in mice, rats... Drug Metab Dispos. 2007 Jan;35(1):51-61. | |||||
| REF 69 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 70 | Clinical pipeline report, company report or official report of Zydus Cadila. | |||||
| REF 71 | Pharma & Vaccines. Product Development Pipeline. April 29 2009. | |||||
| REF 72 | Clinical pipeline report, company report or official report of Tempest Therapeutics. | |||||
| REF 73 | PPAR ligands and their therapeutic applications: a patent review (2008 - 2014).Expert Opin Ther Pat. 2015 Feb;25(2):175-91. | |||||
| REF 74 | Clinical pipeline report, company report or official report of Roche (2009). | |||||
| REF 75 | DOI: 10.1038/scibx.2012.669 | |||||
| REF 76 | The peroxisome proliferator-activated receptor-alpha (PPAR-alpha) agonist, AVE8134, attenuates the progression of heart failure and increases survival in rats. Acta Pharmacol Sin. 2009 Jul;30(7):935-46. | |||||
| REF 77 | Cardiac function and metabolism in Type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-alpha activator. Am J Physiol Heart Circ Physiol. 2002 Sep;283(3):H949-57. | |||||
| REF 78 | Scaffold-based discovery of indeglitazar, a PPAR pan-active anti-diabetic agent. Proc Natl Acad Sci U S A. 2009 Jan 6;106(1):262-7. | |||||
| REF 79 | Identification of a functional peroxisome proliferator-activated receptor (PPAR) response element (PPRE) in the human apolipoprotein A-IV gene. Biochem Pharmacol. 2009 Sep 1;78(5):523-30. | |||||
| REF 80 | A novel PPARalpha agonist ameliorates insulin resistance in dogs fed a high-fat diet. Am J Physiol Endocrinol Metab. 2008 May;294(5):E833-40. | |||||
| REF 81 | KRP-297, MCC-555. Nihon Rinsho. 2001 Nov;59(11):2200-6. | |||||
| REF 82 | Modulation of PPAR receptor subtype selectivity of the ligands: aliphatic chain vs aromatic ring as a spacer between pharmacophore and the lipophilic moiety. Bioorg Med Chem Lett. 2008 Dec 15;18(24):6471-5. | |||||
| REF 83 | Docking and molecular dynamics simulations of peroxisome proliferator activated receptors interacting with pan agonist sodelglitazar. Protein Pept Lett. 2011 Oct;18(10):1021-7. | |||||
| REF 84 | CN patent application no. 100577175, Combination therapy comprising glucose reabsorption inhibitors and PPAR modulators. | |||||
| REF 85 | Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiol. 2010 September; 6(5): 657-691. | |||||
| REF 86 | Antidiabetic and hypolipidemic effects of a novel dual peroxisome proliferator-activated receptor (PPAR) alpha/gamma agonist, E3030, in db/db mice and beagle dogs.J Pharmacol Sci.2008 Sep;108(1):40-8. | |||||
| REF 87 | A peroxisome proliferator-activated receptor alpha/gamma dual agonist with a unique in vitro profile and potent glucose and lipid effects in rodent... Mol Endocrinol. 2005 Jun;19(6):1593-605. | |||||
| REF 88 | CN patent application no. 1882326, Ppar agonists for the treatment of hcv infection. | |||||
| REF 89 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 593). | |||||
| REF 90 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021175) | |||||
| REF 91 | O-arylmandelic acids as highly selective human PPAR alpha/gamma agonists. Bioorg Med Chem Lett. 2003 Oct 6;13(19):3185-90. | |||||
| REF 92 | Fibrates as therapy for osteoarthritis and rheumatoid arthritis A systematic review. Ther Adv Musculoskelet Dis. 2013 February; 5(1): 33-44. | |||||
| REF 93 | US patent application no. 7,816,328, Substituted fused heterocyclic c-glycosides. | |||||
| REF 94 | Peroxisome Proliferators-Activated Receptor (PPAR) Modulators and Metabolic Disorders. PPAR Res. 2008; 2008: 679137. | |||||
| REF 95 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026848) | |||||
| REF 96 | Discovery of cyclic amine-substituted benzoic acids as PPARalpha agonists. Bioorg Med Chem Lett. 2012 Jan 1;22(1):334-8. | |||||
| REF 97 | Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by nitroalkene fatty acids: importance of nitration position and degree ... J Med Chem. 2009 Aug 13;52(15):4631-9. | |||||
| REF 98 | Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995 Oct 13;270(41):23975-83. | |||||
| REF 99 | Discovery of an oxybenzylglycine based peroxisome proliferator activated receptor alpha selective agonist 2-((3-((2-(4-chlorophenyl)-5-methyloxazol... J Med Chem. 2010 Apr 8;53(7):2854-64. | |||||
| REF 100 | Molecular characterization of novel and selective peroxisome proliferator-activated receptor alpha agonists with robust hypolipidemic activity in vivo. Mol Pharmacol. 2009 Feb;75(2):296-306. | |||||
| REF 101 | CN patent application no. 102459215, 3-(4-aminophenyl)-2-furancarboxylic acid derivative and pharmaceutically acceptable salt thereof. | |||||
| REF 102 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 103 | Antidiabetic and hypolipidemic potential of DRF 2519--a dual activator of PPAR-alpha and PPAR-gamma. Eur J Pharmacol. 2004 May 3;491(2-3):195-206. | |||||
| REF 104 | PPAR alpha structure-function relationships derived from species-specific differences in responsiveness to hypolipidemic agents. Biol Chem. 1997 Jul;378(7):651-5. | |||||
| REF 105 | Design and synthesis of a potent and selective triazolone-based peroxisome proliferator-activated receptor alpha agonist. J Med Chem. 2003 Nov 20;46(24):5121-4. | |||||
| REF 106 | PPAR-alpha and -gamma but not -delta agonists inhibit airway inflammation in a murine model of asthma: in vitro evidence for an NF-kappaB-independe... Br J Pharmacol. 2003 May;139(1):163-71. | |||||
| REF 107 | Identification of a subtype selective human PPARalpha agonist through parallel-array synthesis. Bioorg Med Chem Lett. 2001 May 7;11(9):1225-7. | |||||
| REF 108 | Phenylacetic acid derivatives as hPPAR agonists. Bioorg Med Chem Lett. 2003 Apr 7;13(7):1277-80. | |||||
| REF 109 | Design and synthesis of alpha-aryloxy-alpha-methylhydrocinnamic acids: a novel class of dual peroxisome proliferator-activated receptor alpha/gamma agonists. J Med Chem. 2004 May 6;47(10):2422-5. | |||||
| REF 110 | Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 2005 Jun;48(8):1147-53. | |||||
| REF 111 | Pristanic acid and phytanic acid: naturally occurring ligands for the nuclear receptor peroxisome proliferator-activated receptor alpha. J Lipid Res. 2000 Nov;41(11):1801-7. | |||||
| REF 112 | Pharmacological profiles of a novel oral antidiabetic agent, JTT-501, an isoxazolidinedione derivative. Eur J Pharmacol. 1999 Jan 8;364(2-3):211-9. | |||||
| REF 113 | A novel peroxisome proliferator-activated receptor alpha/gamma dual agonist demonstrates favorable effects on lipid homeostasis. Endocrinology. 2004 Apr;145(4):1640-8. | |||||
| REF 114 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||||
| REF 115 | PPARAlpha Ligand-Binding Domain Structures with Endogenous Fatty Acids and Fibrates. iScience. 2020 Oct 23;23(11):101727. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

