Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0LW1Y
|
|||
| Former ID |
DIB001204
|
|||
| Drug Name |
GFT-505
|
|||
| Synonyms |
GFT-1007; PPAR activator (dyslipidemia/type 2 diabetes), Genfit
Click to Show/Hide
|
|||
| Indication | Non-alcoholic steatohepatitis [ICD-11: DB92.1; ICD-10: K75.8; ICD-9: 571.8] | Phase 3 | [1] | |
| Type-2 diabetes [ICD-11: 5A11; ICD-9: 250] | Phase 2 | [2] | ||
| Company |
Genfit SA
|
|||
| Structure |
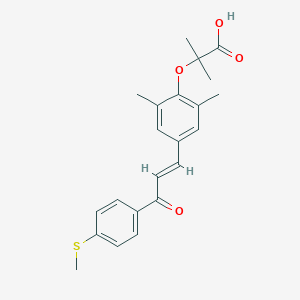 |
Download2D MOL |
||
| Formula |
C22H24O4S
|
|||
| Canonical SMILES |
CC1=CC(=CC(=C1OC(C)(C)C(=O)O)C)C=CC(=O)C2=CC=C(C=C2)SC
|
|||
| InChI |
1S/C22H24O4S/c1-14-12-16(13-15(2)20(14)26-22(3,4)21(24)25)6-11-19(23)17-7-9-18(27-5)10-8-17/h6-13H,1-5H3,(H,24,25)/b11-6+
|
|||
| InChIKey |
AFLFKFHDSCQHOL-IZZDOVSWSA-N
|
|||
| CAS Number |
CAS 923978-27-2
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02704403) Phase 3 Study to Evaluate the Efficacy and Safety of Elafibranor Versus Placebo in Patients With Nonalcoholic Steatohepatitis (NASH) (RESOLVE-IT). U.S. National Institutes of Health. | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025107) | |||
| REF 3 | Dual peroxisome proliferator-activated receptor / agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects.Diabetes Care.2013 Oct;36(10):2923-30. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

