Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04BPS
|
|||
| Former ID |
DIB001531
|
|||
| Drug Name |
Naveglitazar
|
|||
| Synonyms |
LY 519818; LY-818; PPAR alpha/gamma co-agonists, Lilly/Ligand
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Diabetic complication [ICD-11: 5A2Y; ICD-9: 253.5, 588.1] | Phase 2 | [1] | |
| Company |
Ligand Pharmaceuticals
|
|||
| Structure |
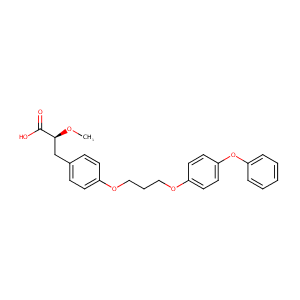 |
Download2D MOL |
||
| Formula |
C25H26O6
|
|||
| Canonical SMILES |
COC(CC1=CC=C(C=C1)OCCCOC2=CC=C(C=C2)OC3=CC=CC=C3)C(=O)O
|
|||
| InChI |
1S/C25H26O6/c1-28-24(25(26)27)18-19-8-10-20(11-9-19)29-16-5-17-30-21-12-14-23(15-13-21)31-22-6-3-2-4-7-22/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1
|
|||
| InChIKey |
OKJHGOPITGTTIM-DEOSSOPVSA-N
|
|||
| CAS Number |
CAS 476436-68-7
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00065312) An Evaluation of an Oral Antidiabetic Agent for the Treatment of Type 2 Diabetes. U.S. National Institutes of Health. | |||
| REF 2 | The disposition and metabolism of naveglitazar, a peroxisome proliferator-activated receptor alpha-gamma dual, gamma-dominant agonist in mice, rats... Drug Metab Dispos. 2007 Jan;35(1):51-61. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

