Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0OW6Z
|
|||
| Former ID |
DIB006861
|
|||
| Drug Name |
Sipoglitazar
|
|||
| Synonyms |
TAK-654
Click to Show/Hide
|
|||
| Indication | Diabetic complication [ICD-11: 5A2Y; ICD-9: 253.5, 588.1] | Terminated | [1] | |
| Company |
Takeda Pharmaceutical Co Ltd
|
|||
| Structure |
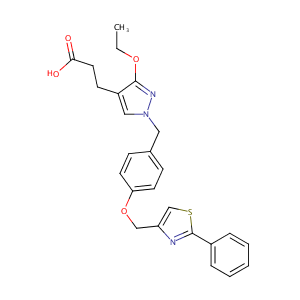 |
Download2D MOL |
||
| Formula |
C25H25N3O4S
|
|||
| Canonical SMILES |
CCOC1=NN(C=C1CCC(=O)O)CC2=CC=C(C=C2)OCC3=CSC(=N3)C4=CC=CC=C4
|
|||
| InChI |
1S/C25H25N3O4S/c1-2-31-24-20(10-13-23(29)30)15-28(27-24)14-18-8-11-22(12-9-18)32-16-21-17-33-25(26-21)19-6-4-3-5-7-19/h3-9,11-12,15,17H,2,10,13-14,16H2,1H3,(H,29,30)
|
|||
| InChIKey |
SRFCAWATPLCLMG-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 342026-92-0
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Pharmacokinetics, safety, and tolerability of saroglitazar (ZYH1), a predominantly PPARalpha agonist with moderate PPARgamma agonist activity in healthy human subjects. Clin Drug Investig. 2013 Nov;33(11):809-16. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

