Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T06273
(Former ID: TTDS00191)
|
|||||
| Target Name |
Poly [ADP-ribose] polymerase 1 (PARP1)
|
|||||
| Synonyms |
Protein poly-ADP-ribosyltransferase PARP1; Poly[ADP-ribose] synthetase-1; Poly[ADP-ribose] synthase 1; Poly(ADP-ribose)polymerase-1; PPOL; PARP-1; NAD(+)Poly [ADP-ribose] polymerase-1 ADP-ribosyltransferase-1; NAD(+) ADP-ribosyltransferase-1; NAD(+) ADP-ribosyltransferase 1; DNA ADP-ribosyltransferase PARP1; ARTD1; ADPRT 1; ADPRT; ADP-ribosyltransferase diphtheria toxin-like 1
Click to Show/Hide
|
|||||
| Gene Name |
PARP1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Acquired cutaneous blood vessel malformation [ICD-11: EF20] | |||||
| 2 | Fallopian tube cancer [ICD-11: 2C74] | |||||
| 3 | Ovarian cancer [ICD-11: 2C73] | |||||
| 4 | Peritoneal cancer [ICD-11: 2C51] | |||||
| Function |
Mainly mediates glutamate and aspartate ADP-ribosylation of target proteins: the ADP-D-ribosyl group of NAD(+) is transferred to the acceptor carboxyl group of glutamate and aspartate residues and further ADP-ribosyl groups are transferred to the 2'-position of the terminal adenosine moiety, building up a polymer with an average chain length of 20-30 units. Mediates the poly(ADP-ribosyl)ation of a number of proteins, including itself, APLF and CHFR. Also mediates serine ADP-ribosylation of target proteins following interaction with HPF1; HPF1 conferring serine specificity. Probably also catalyzes tyrosine ADP-ribosylation of target proteins following interaction with HPF1. Catalyzes the poly-ADP-ribosylation of histones in a HPF1-dependent manner. Involved in the base excision repair (BER) pathway by catalyzing the poly-ADP-ribosylation of a limited number of acceptor proteins involved in chromatin architecture and in DNA metabolism. ADP-ribosylation follows DNA damage and appears as an obligatory step in a detection/signaling pathway leading to the reparation of DNA strand breaks. In addition to base excision repair (BER) pathway, also involved in double-strand breaks (DSBs) repair: together with TIMELESS, accumulates at DNA damage sites and promotes homologous recombination repair by mediating poly-ADP-ribosylation. In addition to proteins, also able to ADP-ribosylate DNA: catalyzes ADP-ribosylation of DNA strand break termini containing terminal phosphates and a 2'-OH group in single- and double-stranded DNA, respectively. Required for PARP9 and DTX3L recruitment to DNA damage sites. PARP1-dependent PARP9-DTX3L-mediated ubiquitination promotes the rapid and specific recruitment of 53BP1/TP53BP1, UIMC1/RAP80, and BRCA1 to DNA damage sites. Acts as a regulator of transcription: positively regulates the transcription of MTUS1 and negatively regulates the transcription of MTUS2/TIP150. With EEF1A1 and TXK, forms a complex that acts as a T-helper 1 (Th1) cell-specific transcription factor and binds the promoter of IFN-gamma to directly regulate its transcription, and is thus involved importantly in Th1 cytokine production. Involved in the synthesis of ATP in the nucleus, together with NMNAT1, PARG and NUDT5. Nuclear ATP generation is required for extensive chromatin remodeling events that are energy-consuming. Poly-ADP-ribosyltransferase that mediates poly-ADP-ribosylation of proteins and plays a key role in DNA repair.
Click to Show/Hide
|
|||||
| BioChemical Class |
Glycosyltransferases
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.4.2.30
|
|||||
| Sequence |
MAESSDKLYRVEYAKSGRASCKKCSESIPKDSLRMAIMVQSPMFDGKVPHWYHFSCFWKV
GHSIRHPDVEVDGFSELRWDDQQKVKKTAEAGGVTGKGQDGIGSKAEKTLGDFAAEYAKS NRSTCKGCMEKIEKGQVRLSKKMVDPEKPQLGMIDRWYHPGCFVKNREELGFRPEYSASQ LKGFSLLATEDKEALKKQLPGVKSEGKRKGDEVDGVDEVAKKKSKKEKDKDSKLEKALKA QNDLIWNIKDELKKVCSTNDLKELLIFNKQQVPSGESAILDRVADGMVFGALLPCEECSG QLVFKSDAYYCTGDVTAWTKCMVKTQTPNRKEWVTPKEFREISYLKKLKVKKQDRIFPPE TSASVAATPPPSTASAPAAVNSSASADKPLSNMKILTLGKLSRNKDEVKAMIEKLGGKLT GTANKASLCISTKKEVEKMNKKMEEVKEANIRVVSEDFLQDVSASTKSLQELFLAHILSP WGAEVKAEPVEVVAPRGKSGAALSKKSKGQVKEEGINKSEKRMKLTLKGGAAVDPDSGLE HSAHVLEKGGKVFSATLGLVDIVKGTNSYYKLQLLEDDKENRYWIFRSWGRVGTVIGSNK LEQMPSKEDAIEHFMKLYEEKTGNAWHSKNFTKYPKKFYPLEIDYGQDEEAVKKLTVNPG TKSKLPKPVQDLIKMIFDVESMKKAMVEYEIDLQKMPLGKLSKRQIQAAYSILSEVQQAV SQGSSDSQILDLSNRFYTLIPHDFGMKKPPLLNNADSVQAKVEMLDNLLDIEVAYSLLRG GSDDSSKDPIDVNYEKLKTDIKVVDRDSEEAEIIRKYVKNTHATTHNAYDLEVIDIFKIE REGECQRYKPFKQLHNRRLLWHGSRTTNFAGILSQGLRIAPPEAPVTGYMFGKGIYFADM VSKSANYCHTSQGDPIGLILLGEVALGNMYELKHASHISKLPKGKHSVKGLGKTTPDPSA NISLDGVDVPLGTGISSGVNDTSLLYNEYIVYDIAQVNLKYLLKLKFNFKTSLW Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A04698 | |||||
| HIT2.0 ID | T73LPO | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 3 Approved Drugs | + | ||||
| 1 | KU-0058948 | Drug Info | Approved | Ovarian cancer | [2] | |
| 2 | Nicotinamide | Drug Info | Approved | Inflammatory skin condition | [3], [4] | |
| 3 | Niraparib Tosylate | Drug Info | Approved | Peritoneal cancer | [5], [6] | |
| Clinical Trial Drug(s) | [+] 8 Clinical Trial Drugs | + | ||||
| 1 | CC-486 | Drug Info | Phase 3 | Myelodysplastic syndrome | [7] | |
| 2 | Nicaraven | Drug Info | Phase 3 | Cerebrovascular disease | [8] | |
| 3 | AG140699 | Drug Info | Phase 2 | Melanoma | [9] | |
| 4 | AZD5305 | Drug Info | Phase 2 | Prostate cancer | [10] | |
| 5 | PMID27841036-Compound-37 | Drug Info | Phase 2 | Ovarian cancer | [11] | |
| 6 | Stenoparib | Drug Info | Phase 2 | Ovarian cancer | [12] | |
| 7 | AMXI 5001 | Drug Info | Phase 1/2 | Solid tumour/cancer | [13] | |
| 8 | NMS-03305293 | Drug Info | Phase 1 | Solid tumour/cancer | [14] | |
| Preclinical Drug(s) | [+] 2 Preclinical Drugs | + | ||||
| 1 | CPH-102 | Drug Info | Preclinical | Solid tumour/cancer | [15] | |
| 2 | PJ34 | Drug Info | Preclinical | Coronavirus infection | [16] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Inhibitor | [+] 128 Inhibitor drugs | + | ||||
| 1 | KU-0058948 | Drug Info | [17] | |||
| 2 | CC-486 | Drug Info | [19] | |||
| 3 | AG140699 | Drug Info | [20] | |||
| 4 | AZD5305 | Drug Info | [21] | |||
| 5 | PMID27841036-Compound-37 | Drug Info | [22] | |||
| 6 | Stenoparib | Drug Info | [12] | |||
| 7 | AMXI 5001 | Drug Info | [23] | |||
| 8 | NMS-03305293 | Drug Info | [24] | |||
| 9 | 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 1 | Drug Info | [22] | |||
| 10 | 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 2 | Drug Info | [22] | |||
| 11 | 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 3 | Drug Info | [22] | |||
| 12 | 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 4 | Drug Info | [22] | |||
| 13 | 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 5 | Drug Info | [22] | |||
| 14 | 3-phenyl isoquinolin-1(2H) derivative 1 | Drug Info | [22] | |||
| 15 | 3-phenyl isoquinolin-1(2H) derivative 2 | Drug Info | [22] | |||
| 16 | 4-Carboxamido-isoindolinone derivative 1 | Drug Info | [22] | |||
| 17 | 4-Carboxamido-isoindolinone derivative 2 | Drug Info | [22] | |||
| 18 | 4-Carboxamido-isoindolinone derivative 3 | Drug Info | [22] | |||
| 19 | 4-Carboxamido-isoindolinone derivative 4 | Drug Info | [22] | |||
| 20 | 4-Carboxamido-isoindolinone derivative 5 | Drug Info | [22] | |||
| 21 | 7-azaindole derivative 8 | Drug Info | [22] | |||
| 22 | Benzimidazole carboxamide derivative 1 | Drug Info | [22] | |||
| 23 | Dihydrodiazepinocarbazolone derivative 1 | Drug Info | [22] | |||
| 24 | Dihydropyrido phthalazinone derivative 1 | Drug Info | [22] | |||
| 25 | Dihydropyrido phthalazinone derivative 2 | Drug Info | [22] | |||
| 26 | Phthalazine derivative 3 | Drug Info | [22] | |||
| 27 | Phthalazine ketone derivative 1 | Drug Info | [22] | |||
| 28 | Phthalazine ketone derivative 2 | Drug Info | [22] | |||
| 29 | Phthalazine ketone derivative 3 | Drug Info | [22] | |||
| 30 | PMID27841036-Compound-33 | Drug Info | [22] | |||
| 31 | Quinazolinedione derivative 1 | Drug Info | [22] | |||
| 32 | Quinazolinedione derivative 2 | Drug Info | [22] | |||
| 33 | Quinazolinedione derivative 3 | Drug Info | [22] | |||
| 34 | Tetra-cyclic pyridophthalazinone derivative 1 | Drug Info | [22] | |||
| 35 | Tetra-hydro-quinoline derivative 1 | Drug Info | [22] | |||
| 36 | Tricyclic indole compound 13 | Drug Info | [22] | |||
| 37 | CPH-102 | Drug Info | [25] | |||
| 38 | PJ34 | Drug Info | [26] | |||
| 39 | NU1025 | Drug Info | [27] | |||
| 40 | (E)-N-(4-Phenylthiazol-2-yl) cinnamamide | Drug Info | [28] | |||
| 41 | 1,2,3,4,4a,5-hexahydrophenanthridin-6(10bH)-one | Drug Info | [28] | |||
| 42 | 1,7,8,9-tetrahydro-1,5-diaza-trindene-4,6-dione | Drug Info | [29] | |||
| 43 | 2,3-dihydro-1H-benzo[de]isoquinolin-1-one | Drug Info | [30] | |||
| 44 | 2,8-Dimethyl-3H-quinazolin-4-one | Drug Info | [31] | |||
| 45 | 2-(2-Chlorophenyl)-2H-indazole-7-carboxamide | Drug Info | [32] | |||
| 46 | 2-(3'-Methoxyphenyl) Benzimidazole-4-Carboxamide | Drug Info | [27] | |||
| 47 | 2-(3-Chlorophenyl)-2H-indazole-7-carboxamide | Drug Info | [32] | |||
| 48 | 2-(3-Piperidin-1-yl-propyl)-3H-quinazolin-4-one | Drug Info | [33] | |||
| 49 | 2-(4-Amino-phenyl)-8-hydroxy-3H-quinazolin-4-one | Drug Info | [31] | |||
| 50 | 2-(4-Amino-phenyl)-8-methyl-3H-quinazolin-4-one | Drug Info | [31] | |||
| 51 | 2-(4-Azido-phenyl)-8-methoxy-3H-quinazolin-4-one | Drug Info | [31] | |||
| 52 | 2-(4-Chlorophenyl)-2H-indazole-7-carboxamide | Drug Info | [32] | |||
| 53 | 2-(4-Chlorophenyl)-5-Quinoxalinecarboxamide | Drug Info | [34] | |||
| 54 | 2-(4-Hydroxy-phenyl)-8-methyl-3H-quinazolin-4-one | Drug Info | [31] | |||
| 55 | 2-(4-Methoxy-phenyl)-8-methyl-3H-quinazolin-4-one | Drug Info | [31] | |||
| 56 | 2-(4-methoxyphenyl)quinoline-8-carboxamide | Drug Info | [35] | |||
| 57 | 2-Benzyl-2H-indazole-7-carboxamide | Drug Info | [32] | |||
| 58 | 2-ethylquinoline-8-carboxamide | Drug Info | [35] | |||
| 59 | 2-Methylquinoline-8-carboxamide | Drug Info | [35] | |||
| 60 | 2-phenyl-2H-benzo[d][1,2,3]triazole-4-carboxamide | Drug Info | [32] | |||
| 61 | 2-phenyl-2H-indazole-7-carboxamide | Drug Info | [32] | |||
| 62 | 2-phenylpyrazolo-[1,5-a]pyridine-7-carboxamide | Drug Info | [32] | |||
| 63 | 2-phenylquinoline-8-carboxamide | Drug Info | [35] | |||
| 64 | 2H-Isoquinolin-1-one | Drug Info | [31] | |||
| 65 | 3-(4-aminophenyl)quinoxaline-5-carboxamide | Drug Info | [28] | |||
| 66 | 3-(4-cyanophenyl)quinoxaline-5-carboxamide | Drug Info | [28] | |||
| 67 | 3-(4-methoxyphenyl)quinoxaline-5-carboxamide | Drug Info | [28] | |||
| 68 | 3-aminobenzamide | Drug Info | [26], [31] | |||
| 69 | 3-aminobenzo[c][1,5]naphthyridin-6(5H)-one | Drug Info | [28] | |||
| 70 | 3-Ethenylquinoline-8-carboxamide | Drug Info | [35] | |||
| 71 | 3-Ethylquinoline-8-carboxamide | Drug Info | [35] | |||
| 72 | 3-Ethynylquinoline-8-carboxamide | Drug Info | [35] | |||
| 73 | 3-Hydroxy-benzamide | Drug Info | [31] | |||
| 74 | 3-Methoxybenzamide | Drug Info | [34] | |||
| 75 | 3-Methylquinoline-8-carboxamide | Drug Info | [35] | |||
| 76 | 3-Morpholin-4-ylmethyl-5H-phenanthridin-6-one | Drug Info | [36] | |||
| 77 | 3-Phenylquinoline-8-carboxamide | Drug Info | [35] | |||
| 78 | 3-Prop-1-ynylquinoline-8-carboxamide | Drug Info | [35] | |||
| 79 | 4-(4-Morpholin-4-yl-butyl)-2H-phthalazin-1-one | Drug Info | [36] | |||
| 80 | 4-(5-Morpholin-4-yl-pentyl)-2H-phthalazin-1-one | Drug Info | [36] | |||
| 81 | 4-amino-1,8-naphthalimide | Drug Info | [27], [29] | |||
| 82 | 4-benzylphthalazin-1(2H)-one | Drug Info | [28] | |||
| 83 | 4-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione | Drug Info | [29] | |||
| 84 | 5-amino-3,4-dihydroisoquinolin-1(2H)-one | Drug Info | [30] | |||
| 85 | 5-aminoisoquinolin-1(2H)-one | Drug Info | [35] | |||
| 86 | 5-Chloro-2-methyl-3H-quinazolin-4-one | Drug Info | [33] | |||
| 87 | 5-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione | Drug Info | [29] | |||
| 88 | 8-Amino-6H,11H-indeno[1,2-c]isoquinolin-5-one | Drug Info | [37] | |||
| 89 | 8-Fluoro-6H,11H-indeno[1,2-c]isoquinolin-5-one | Drug Info | [37] | |||
| 90 | 8-Hydroxy-2-(4-nitro-phenyl)-3H-quinazolin-4-one | Drug Info | [31] | |||
| 91 | 8-Hydroxy-2-phenyl-3H-quinazolin-4-one | Drug Info | [31] | |||
| 92 | 8-Methoxy-2-(4-nitro-phenyl)-3H-quinazolin-4-one | Drug Info | [31] | |||
| 93 | 8-Methoxy-2-methyl-3H-quinazolin-4-one | Drug Info | [31] | |||
| 94 | 8-Methoxy-2-phenyl-3H-quinazolin-4-one | Drug Info | [31] | |||
| 95 | 8-Methyl-2-(4-nitro-phenyl)-3H-quinazolin-4-one | Drug Info | [31] | |||
| 96 | 8-Methyl-2-phenyl-3H-quinazolin-4-one | Drug Info | [31] | |||
| 97 | 8-Nitro-6H,11H-indeno[1,2-c]isoquinolin-5-one | Drug Info | [37] | |||
| 98 | 9-Amino-6H,11H-indeno[1,2-c]isoquinolin-5-one | Drug Info | [37] | |||
| 99 | 9-Fluoro-6H,11H-indeno[1,2-c]isoquinolin-5-one | Drug Info | [37] | |||
| 100 | A-620223 | Drug Info | [25] | |||
| 101 | AG-014376 | Drug Info | [38] | |||
| 102 | ANG-2684 | Drug Info | [25] | |||
| 103 | ANG-2864 | Drug Info | [25] | |||
| 104 | Benzo[c][1,5]naphthyridin-6(5H)-one | Drug Info | [28] | |||
| 105 | BPI-704001 | Drug Info | [25] | |||
| 106 | BZ3 | Drug Info | [39] | |||
| 107 | BZ5 | Drug Info | [39] | |||
| 108 | BZ6 | Drug Info | [39] | |||
| 109 | Carba-Nicotinamide-Adenine-Dinucleotide | Drug Info | [34] | |||
| 110 | CEP-6800 | Drug Info | [40] | |||
| 111 | DR2313 | Drug Info | [20] | |||
| 112 | EB-47 | Drug Info | [20] | |||
| 113 | HYDAMTIQ | Drug Info | [25] | |||
| 114 | INO-1002 | Drug Info | [25] | |||
| 115 | KR-33889 | Drug Info | [25] | |||
| 116 | KU-58684 | Drug Info | [28] | |||
| 117 | ME0328 | Drug Info | [41] | |||
| 118 | N-(4-Phenylthiazol-2-yl)isonicotinamide | Drug Info | [28] | |||
| 119 | PD-128763 | Drug Info | [31], [34] | |||
| 120 | Pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione | Drug Info | [29] | |||
| 121 | Pyrrolo[3,4-e]indole-1,3(2H,6H)-dione | Drug Info | [29] | |||
| 122 | Quinoline-8-carboxamide | Drug Info | [35] | |||
| 123 | S-070 | Drug Info | [25] | |||
| 124 | S-111 | Drug Info | [25] | |||
| 125 | Thieno-phenanthridin-6-one | Drug Info | [20] | |||
| 126 | TI3 | Drug Info | [39] | |||
| 127 | TI4 | Drug Info | [39] | |||
| 128 | [2(R,S)-2-Sulfanylheptanoyl]-Phe-Ala | Drug Info | [39] | |||
| Binder | [+] 1 Binder drugs | + | ||||
| 1 | Nicotinamide | Drug Info | [1] | |||
| Modulator | [+] 2 Modulator drugs | + | ||||
| 1 | Niraparib Tosylate | Drug Info | [18] | |||
| 2 | Nicaraven | Drug Info | [8] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Rucaparib | Ligand Info | |||||
| Structure Description | Crystal Structure of human PARP-1 CAT domain bound to inhibitor rucaparib | PDB:6VKK | ||||
| Method | X-ray diffraction | Resolution | 2.10 Å | Mutation | Yes | [42] |
| PDB Sequence |
KSKLPKPVQD
671 LIKMIFDVES681 MKKAMVEYEI691 DLQKMPLGKL701 SKRQIQAAYS711 ILSEVQQAVS 721 QGSSDSQILD731 LSNRFYTLIP741 HDFGMKKPPL751 LNNADSVQAK761 AEMLDNLLDI 771 EVAYSLLRGS786 KDPIDVNYEK796 LKTDIKVVDR806 DSEEAEIIRK816 YVKNTHATTH 826 NAYDLEVIDI836 FKIEREGECQ846 RYKPFKQLHN856 RRLLWHGSRT866 TNFAGILSQG 876 LRIAPPEAPV886 TGYMFGKGIY896 FADMVSKSAN906 YCHTSQGDPI916 GLILLGEVAL 926 GNMYELKHAS936 HISKLPKGKH946 SVKGLGKTTP956 DPSANISLDG966 VDVPLGTGIS 976 SGVNDTSLLY986 NEYIVYDIAQ996 VNLKYLLKLK1006 FNFKT
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: Olaparib | Ligand Info | |||||
| Structure Description | Structure of the catalytic domain of PARP1 in complex with olaparib | PDB:7KK4 | ||||
| Method | X-ray diffraction | Resolution | 1.96 Å | Mutation | No | [43] |
| PDB Sequence |
KSKLPKPVQD
671 LIKMIFDVES681 MKKAMVEYEI691 DLQKMPLGKL701 SKRQIQAAYS711 ILSEVQQAVS 721 QGSSDSQILD731 LSNRFYTLIP741 HDFPPLLNNA755 DSVQAKVEML765 DNLLDIEVAY 775 SLLRGSKDPI790 DVNYEKLKTD800 IKVVDRDSEE810 AEIIRKYVKN820 THATTHNAYD 830 LEVIDIFKIE840 REGECQRYKP850 FKQLHNRRLL860 WHGSRTTNFA870 GILSQGLRIA 880 PPEAPVTGYM890 FGKGIYFADM900 VSKSANYCHT910 SQGDPIGLIL920 LGEVALGNMY 930 ELKHASHISK940 LPKGKHSVKG950 LGKTTPDPSA960 NISLDGVDVP970 LGTGISSGVN 980 DTSLLYNEYI990 VYDIAQVNLK1000 YLLKLKFNFK1010
|
|||||
|
|
TYR710
3.472
ASP766
3.307
LEU769
3.681
ASP770
4.048
TRP861
3.770
HIS862
3.294
GLY863
2.800
ARG878
2.978
ILE879
4.112
ALA880
3.698
PRO881
3.728
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
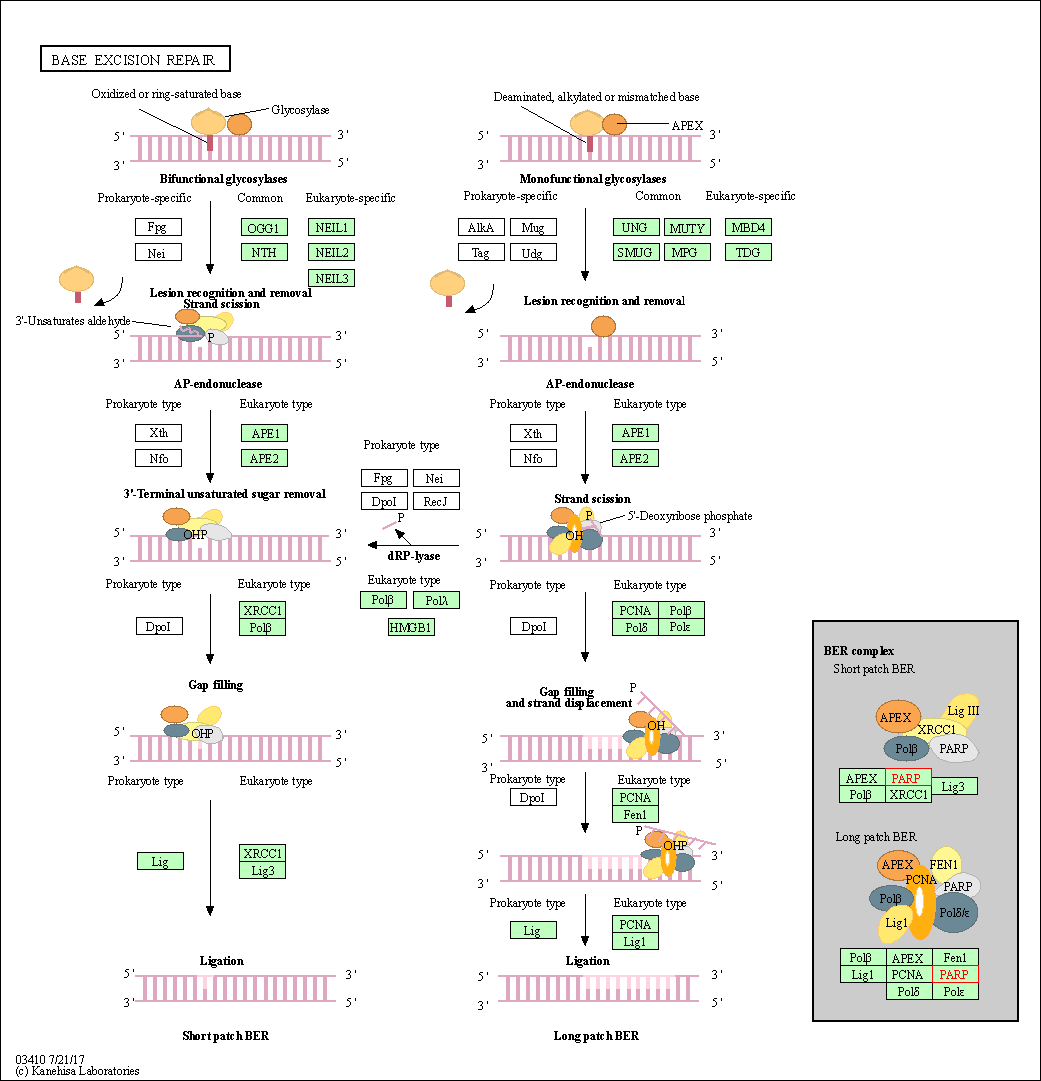
| Base excision repair | hsa03410 | Affiliated Target |

|
| Class: Genetic Information Processing => Replication and repair | Pathway Hierarchy | ||
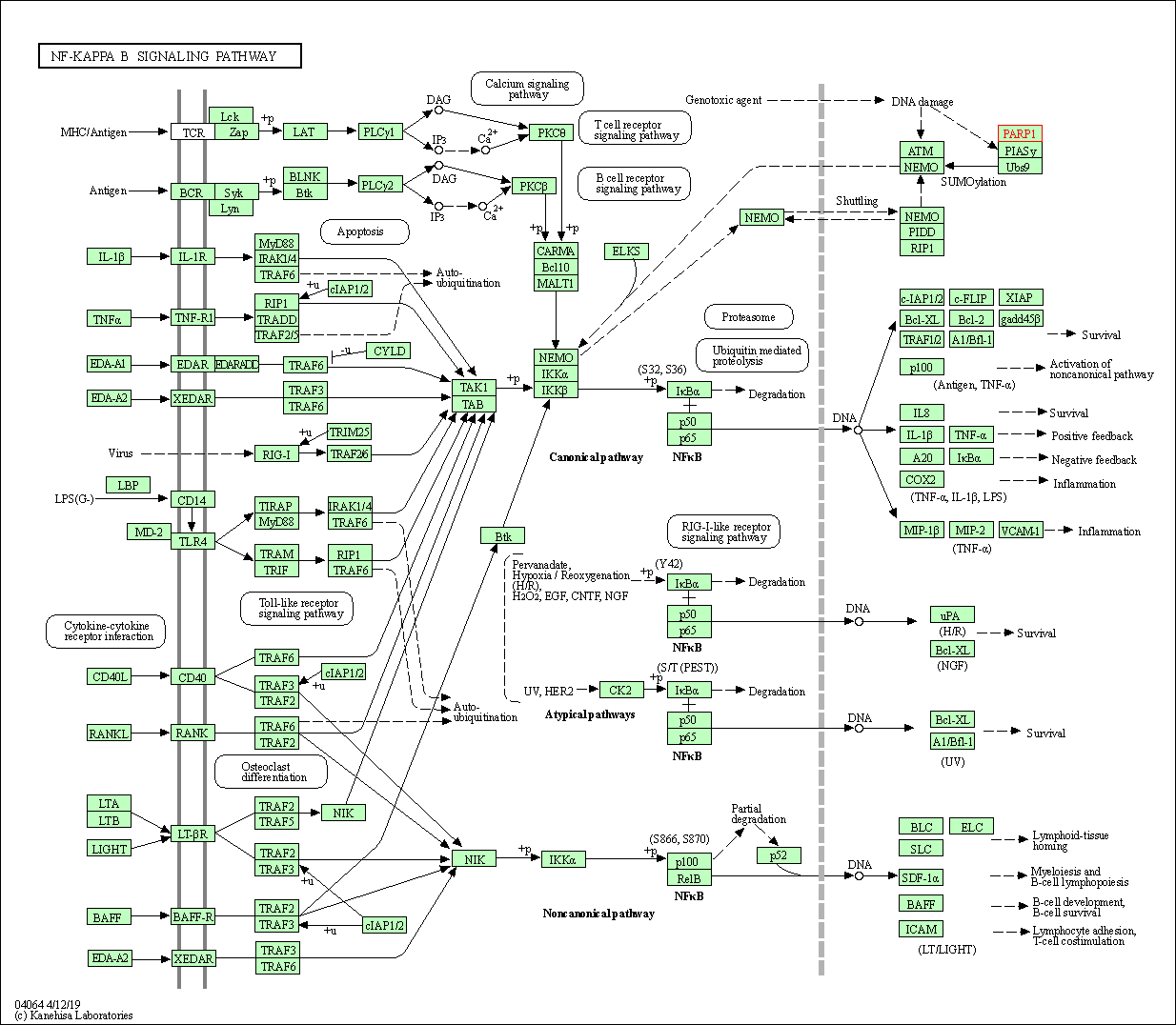
| NF-kappa B signaling pathway | hsa04064 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
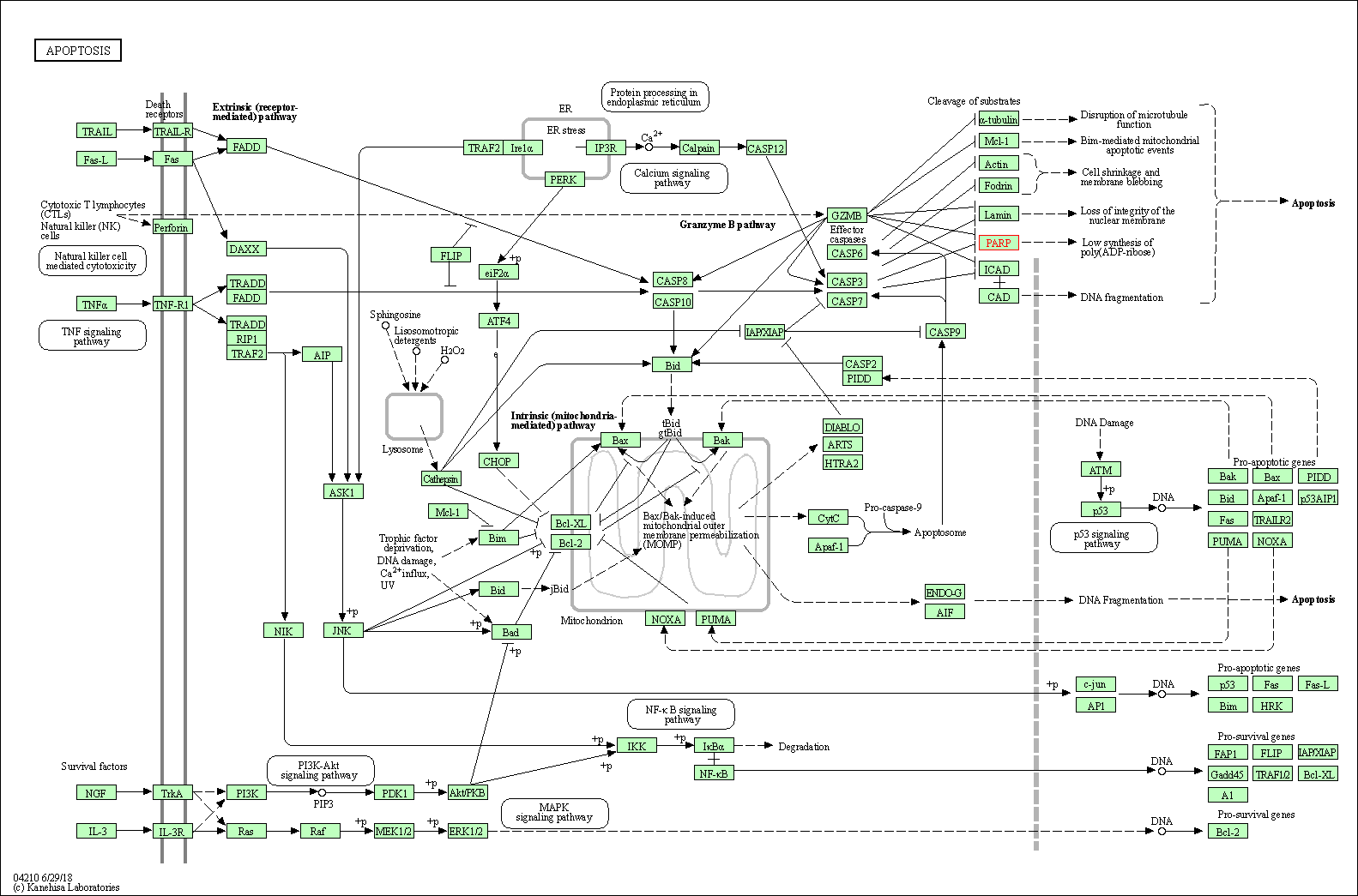
| Apoptosis | hsa04210 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
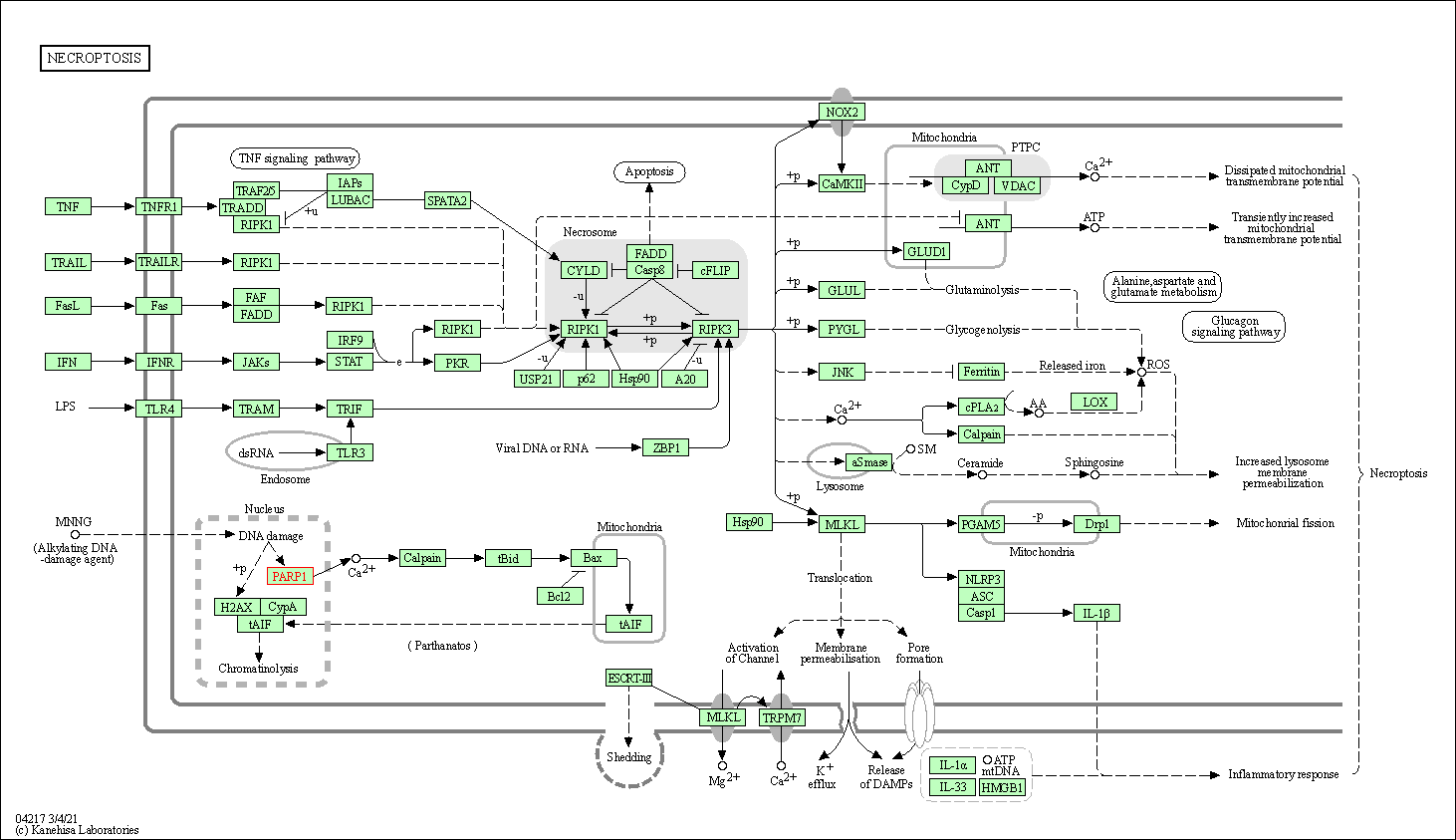
| Necroptosis | hsa04217 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Degree | 25 | Degree centrality | 2.69E-03 | Betweenness centrality | 9.89E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.43E-01 | Radiality | 1.43E+01 | Clustering coefficient | 1.37E-01 |
| Neighborhood connectivity | 3.99E+01 | Topological coefficient | 7.01E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 2 KEGG Pathways | + | ||||
| 1 | Base excision repair | |||||
| 2 | NF-kappa B signaling pathway | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | FAS signaling pathway | |||||
| PID Pathway | [+] 3 PID Pathways | + | ||||
| 1 | Integrin-linked kinase signaling | |||||
| 2 | Caspase Cascade in Apoptosis | |||||
| 3 | Notch-mediated HES/HEY network | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | Dual Incision in GG-NER | |||||
| WikiPathways | [+] 4 WikiPathways | + | ||||
| 1 | FAS pathway and Stress induction of HSP regulation | |||||
| 2 | Transcriptional activity of SMAD2/SMAD3:SMAD4 heterotrimer | |||||
| 3 | Nanoparticle triggered regulated necrosis | |||||
| 4 | Corticotropin-releasing hormone | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | beta-1,2,3-Triazolyl-nucleosides as nicotinamide riboside mimics. Nucleosides Nucleotides Nucleic Acids. 2009 Mar;28(3):238-59. | |||||
| REF 2 | 2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1588). | |||||
| REF 4 | Drug information of Nicotinamide. United States Environmental Protection Agency. 2015 | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2018 | |||||
| REF 6 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||||
| REF 7 | J Clin Oncol 33, 2015 (suppl, abstr TPS7097). | |||||
| REF 8 | Inhibition of poly (ADP-ribose) polymerase as a protective effect of nicaraven in ionizing radiation- and ara-C-induced cell death. Anticancer Res. 2006 Sep-Oct;26(5A):3421-7. | |||||
| REF 9 | Critical Care Nephrology, Claudio Ronco,Rinaldo Bellomo,John A. Kellum. Page(444). | |||||
| REF 10 | ClinicalTrials.gov (NCT05367440) A Multi-arm, Open-label Phase I/IIa Study to Assess the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Preliminary Efficacy of AZD5305 in Combination With New Hormonal Agents in Patients With Metastatic Prostate Cancer (PETRANHA). U.S.National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT03878849) Investigation of 2X-121 in Patients With Advanced Ovarian Cancer Selected by the 2X-121 DRP (PREDICT 2X-121). U.S. National Institutes of Health. | |||||
| REF 12 | Clinical pipeline report, company report or official report of Allarity Therapeutics. | |||||
| REF 13 | ClinicalTrials.gov (NCT04503265) A Trial of AMXI-5001 for Treatment in Patients With Advanced Malignancies. U.S. National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT04182516) Study of NMS-03305293 in Pts With Selected Advanced/Metastatic Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006586) | |||||
| REF 16 | Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47. | |||||
| REF 17 | Structural basis for inhibitor specificity in human poly(ADP-ribose) polymerase-3. J Med Chem. 2009 May 14;52(9):3108-11. | |||||
| REF 18 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 19 | Tricyclic benzimidazoles as potent poly(ADP-ribose) polymerase-1 inhibitors. J Med Chem. 2003 Jan 16;46(2):210-3. | |||||
| REF 20 | Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005 May;4(5):421-40. | |||||
| REF 21 | Preclinical Characterization of AZD5305, A Next-Generation, Highly Selective PARP1 Inhibitor and Trapper. Clin Cancer Res. 2022 Nov 1;28(21):4724-4736. | |||||
| REF 22 | PARP inhibitors as antitumor agents: a patent update (2013-2015).Expert Opin Ther Pat. 2017 Mar;27(3):363-382. | |||||
| REF 23 | AMXI-5001, a novel dual parp1/2 and microtubule polymerization inhibitor for the treatment of human cancers. Am J Cancer Res. 2020 Aug 1;10(8):2649-2676. | |||||
| REF 24 | NMS-P293, a PARP-1 selective inhibitor with no trapping activity and high CNS penetration, possesses potent in vivo efficacy and represents a novel therapeutic option for brain localized metastases and glioblastoma. Cancer Res 2018;78(13 Suppl):Abstract nr 4843. | |||||
| REF 25 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2771). | |||||
| REF 26 | Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001 Jan;7(1):108-13. | |||||
| REF 27 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 28 | Design, synthesis, and cytoprotective effect of 2-aminothiazole analogues as potent poly(ADP-ribose) polymerase-1 inhibitors. J Med Chem. 2009 Feb 12;52(3):718-25. | |||||
| REF 29 | Synthesis and structure-activity relationships of novel poly(ADP-ribose) polymerase-1 inhibitors. Bioorg Med Chem Lett. 2006 Feb 15;16(4):938-42. | |||||
| REF 30 | Discovery and SAR of novel, potent and selective hexahydrobenzonaphthyridinone inhibitors of poly(ADP-ribose)polymerase-1 (PARP-1). Bioorg Med Chem Lett. 2010 Jan 15;20(2):448-52. | |||||
| REF 31 | Resistance-modifying agents. 5. Synthesis and biological properties of quinazolinone inhibitors of the DNA repair enzyme poly(ADP-ribose) polymeras... J Med Chem. 1998 Dec 17;41(26):5247-56. | |||||
| REF 32 | Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor effica... J Med Chem. 2009 Nov 26;52(22):7170-85. | |||||
| REF 33 | Rational approaches to discovery of orally active and brain-penetrable quinazolinone inhibitors of poly(ADP-ribose)polymerase. J Med Chem. 2004 Aug 12;47(17):4151-4. | |||||
| REF 34 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 35 | Design, synthesis, and evaluation in vitro of quinoline-8-carboxamides, a new class of poly(adenosine-diphosphate-ribose)polymerase-1 (PARP-1) inhi... J Med Chem. 2009 Feb 12;52(3):868-77. | |||||
| REF 36 | 4-Phenyl-1,2,3,6-tetrahydropyridine, an excellent fragment to improve the potency of PARP-1 inhibitors. Bioorg Med Chem Lett. 2005 Oct 1;15(19):4221-5. | |||||
| REF 37 | Discovery of potent poly(ADP-ribose) polymerase-1 inhibitors from the modification of indeno[1,2-c]isoquinolinone. J Med Chem. 2005 Aug 11;48(16):5100-3. | |||||
| REF 38 | Design, synthesis, and evaluation of 3,4-dihydro-2H-[1,4]diazepino[6,7,1-hi]indol-1-ones as inhibitors of poly(ADP-ribose) polymerase. J Med Chem. 2004 Oct 21;47(22):5467-81. | |||||
| REF 39 | Identification of potent nontoxic poly(ADP-Ribose) polymerase-1 inhibitors: chemopotentiation and pharmacological studies. Clin Cancer Res. 2003 Jul;9(7):2711-8. | |||||
| REF 40 | Novel poly(ADP-ribose) polymerase-1 inhibitors. Bioorg Med Chem Lett. 2007 Jan 15;17(2):542-5. | |||||
| REF 41 | Chemical probes to study ADP-ribosylation: synthesis and biochemical evaluation of inhibitors of the human ADP-ribosyltransferase ARTD3/PARP3. J Med Chem. 2013 Dec 12;56(23):9556-68. | |||||
| REF 42 | Structural basis for allosteric PARP-1 retention on DNA breaks. Science. 2020 Apr 3;368(6486):eaax6367. | |||||
| REF 43 | Dissecting the molecular determinants of clinical PARP1 inhibitor selectivity for tankyrase1. J Biol Chem. 2021 Jan-Jun;296:100251. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

