Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T86350
(Former ID: TTDR01265)
|
|||||
| Target Name |
Erbb3 tyrosine kinase receptor (Erbb-3)
|
|||||
| Synonyms |
Tyrosine kinase-type cell surface receptor HER3; Receptor tyrosine-protein kinase erbB-3; Proto-oncogene-like protein c-ErbB-3; HER3; C-erbB3; C-erbB-3 protein
Click to Show/Hide
|
|||||
| Gene Name |
ERBB3
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 10 Target-related Diseases | + | ||||
| 1 | Colorectal cancer [ICD-11: 2B91] | |||||
| 2 | Head and neck cancer [ICD-11: 2D42] | |||||
| 3 | Lung cancer [ICD-11: 2C25] | |||||
| 4 | Pancreatic cancer [ICD-11: 2C10] | |||||
| 5 | Psoriasis [ICD-11: EA90] | |||||
| 6 | Squamous cell carcinoma [ICD-11: 2B60-2D01] | |||||
| 7 | Breast cancer [ICD-11: 2C60-2C6Y] | |||||
| 8 | Malignant mesenchymal neoplasm [ICD-11: 2B5D-2B5Y] | |||||
| 9 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| 10 | Metastatic lymph node neoplasm [ICD-11: 2D60] | |||||
| Function |
Binds to neuregulin-1 (NRG1) and is activated by it; ligand-binding increases phosphorylation on tyrosine residues and promotes its association with the p85 subunit of phosphatidylinositol 3-kinase. May also be activated by CSPG5. Involved in the regulation of myeloid cell differentiation. Tyrosine-protein kinase that plays an essential role as cell surface receptor for neuregulins.
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.10.1
|
|||||
| Sequence |
MRANDALQVLGLLFSLARGSEVGNSQAVCPGTLNGLSVTGDAENQYQTLYKLYERCEVVM
GNLEIVLTGHNADLSFLQWIREVTGYVLVAMNEFSTLPLPNLRVVRGTQVYDGKFAIFVM LNYNTNSSHALRQLRLTQLTEILSGGVYIEKNDKLCHMDTIDWRDIVRDRDAEIVVKDNG RSCPPCHEVCKGRCWGPGSEDCQTLTKTICAPQCNGHCFGPNPNQCCHDECAGGCSGPQD TDCFACRHFNDSGACVPRCPQPLVYNKLTFQLEPNPHTKYQYGGVCVASCPHNFVVDQTS CVRACPPDKMEVDKNGLKMCEPCGGLCPKACEGTGSGSRFQTVDSSNIDGFVNCTKILGN LDFLITGLNGDPWHKIPALDPEKLNVFRTVREITGYLNIQSWPPHMHNFSVFSNLTTIGG RSLYNRGFSLLIMKNLNVTSLGFRSLKEISAGRIYISANRQLCYHHSLNWTKVLRGPTEE RLDIKHNRPRRDCVAEGKVCDPLCSSGGCWGPGPGQCLSCRNYSRGGVCVTHCNFLNGEP REFAHEAECFSCHPECQPMEGTATCNGSGSDTCAQCAHFRDGPHCVSSCPHGVLGAKGPI YKYPDVQNECRPCHENCTQGCKGPELQDCLGQTLVLIGKTHLTMALTVIAGLVVIFMMLG GTFLYWRGRRIQNKRAMRRYLERGESIEPLDPSEKANKVLARIFKETELRKLKVLGSGVF GTVHKGVWIPEGESIKIPVCIKVIEDKSGRQSFQAVTDHMLAIGSLDHAHIVRLLGLCPG SSLQLVTQYLPLGSLLDHVRQHRGALGPQLLLNWGVQIAKGMYYLEEHGMVHRNLAARNV LLKSPSQVQVADFGVADLLPPDDKQLLYSEAKTPIKWMALESIHFGKYTHQSDVWSYGVT VWELMTFGAEPYAGLRLAEVPDLLEKGERLAQPQICTIDVYMVMVKCWMIDENIRPTFKE LANEFTRMARDPPRYLVIKRESGPGIAPGPEPHGLTNKKLEEVELEPELDLDLDLEAEED NLATTTLGSALSLPVGTLNRPRGSQSLLSPSSGYMPMNQGNLGESCQESAVSGSSERCPR PVSLHPMPRGCLASESSEGHVTGSEAELQEKVSMCRSRSRSRSPRPRGDSAYHSQRHSLL TPVTPLSPPGLEEEDVNGYVMPDTHLKGTPSSREGTLSSVGLSSVLGTEEEDEDEEYEYM NRRRRHSPPHPPRPSSLEELGYEYMDVGSDLSASLGSTQSCPLHPVPIMPTAGTTPDEDY EYMNRQRDGGGPGGDYAAMGACPASEQGYEEMRAFQGPGHQAPHVHYARLKTLRSLEATD SAFDNPDYWHSRLFPKANAQRT Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T14JZK | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 22 Clinical Trial Drugs | + | ||||
| 1 | CDX-3379 | Drug Info | Phase 2 | Squamous cell carcinoma | [2] | |
| 2 | Elisidepsin | Drug Info | Phase 2 | Psoriasis vulgaris | [3] | |
| 3 | MEHD-7945A | Drug Info | Phase 2 | Colorectal cancer | [4], [5] | |
| 4 | MM-121 | Drug Info | Phase 2 | Breast cancer | [6] | |
| 5 | MM-141 | Drug Info | Phase 2 | Solid tumour/cancer | [7] | |
| 6 | Seribantumab | Drug Info | Phase 2 | Colorectal cancer | [8] | |
| 7 | Zenocutuzumab | Drug Info | Phase 2 | Pancreatic cancer | [9] | |
| 8 | AMG888/U3-1287 | Drug Info | Phase 1/2 | Non-small-cell lung cancer | [10] | |
| 9 | LJM716 | Drug Info | Phase 1/2 | Breast cancer | [11] | |
| 10 | Sym013 | Drug Info | Phase 1/2 | Epithelial ovarian cancer | [2] | |
| 11 | Zenocutuzomab | Drug Info | Phase 1/2 | Solid tumour/cancer | [12] | |
| 12 | Anti-HER3/EGFR DAF | Drug Info | Phase 1 | Metastatic epithelial tumour | [13] | |
| 13 | AV-203 | Drug Info | Phase 1 | Solid tumour/cancer | [14] | |
| 14 | Drug 2849330 | Drug Info | Phase 1 | Solid tumour/cancer | [15] | |
| 15 | GSK2849330 | Drug Info | Phase 1 | Solid tumour/cancer | [15] | |
| 16 | KTN3379 | Drug Info | Phase 1 | Solid tumour/cancer | [16] | |
| 17 | MM-151 | Drug Info | Phase 1 | Solid tumour/cancer | [17] | |
| 18 | Patritumab | Drug Info | Phase 1 | Non-small-cell lung cancer | [18] | |
| 19 | Recombinant human Erbb3 fragment therapeutic tumor vaccine | Drug Info | Phase 1 | Solid tumour/cancer | [19] | |
| 20 | REGN1400 | Drug Info | Phase 1 | Solid tumour/cancer | [20] | |
| 21 | RG7116 | Drug Info | Phase 1 | Solid tumour/cancer | [21] | |
| 22 | SI-B001 | Drug Info | Phase 1 | Metastatic epithelial tumour | [22] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Antagonist | [+] 2 Antagonist drugs | + | ||||
| 1 | CDX-3379 | Drug Info | [8] | |||
| 2 | Seribantumab | Drug Info | [8] | |||
| Inhibitor | [+] 4 Inhibitor drugs | + | ||||
| 1 | Elisidepsin | Drug Info | [1] | |||
| 2 | Sym013 | Drug Info | [2] | |||
| 3 | Zenocutuzomab | Drug Info | [30] | |||
| 4 | Soluble ErbB3 theragnostics | Drug Info | [28] | |||
| Modulator | [+] 8 Modulator drugs | + | ||||
| 1 | MEHD-7945A | Drug Info | [23], [24] | |||
| 2 | MM-121 | Drug Info | [25] | |||
| 3 | MM-141 | Drug Info | [26] | |||
| 4 | AMG888/U3-1287 | Drug Info | [28] | |||
| 5 | Anti-HER3/EGFR DAF | Drug Info | [23] | |||
| 6 | MM-151 | Drug Info | [26] | |||
| 7 | RG7116 | Drug Info | [38] | |||
| 8 | SYM-011 | Drug Info | [28] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Bosutinib | Ligand Info | |||||
| Structure Description | HER3 pseudokinase domain bound to bosutinib | PDB:6OP9 | ||||
| Method | X-ray diffraction | Resolution | 2.50 Å | Mutation | No | [40] |
| PDB Sequence |
VLARIFKETE
689 LRKLKVLGSG699 VFGTVHKGVW709 IPEGESIKIP719 VCIKVIEDKS729 GRQSFQAVTD 739 HMLAIGSLDH749 AHIVRLLGLC759 PGSSLQLVTQ769 YLPLGSLLDH779 VRQHRGALGP 789 QLLLNWGVQI799 AKGMYYLEEH809 GMVHRNLAAR819 NVLLKSPSQV829 QVADFGVADL 839 LPPDDKQAKT854 PIKWMALESI864 HFGKYTHQSD874 VWSYGVTVWE884 LMTFGAEPYA 894 GLRLAEVPDL904 LEKGERLAQP914 QICTIDVYMV924 MVKCWMIDEN934 IRPTFKELAN 944 EFTRMARDPP954 RYLVIK
|
|||||
|
|
LEU696
3.423
GLY697
4.357
VAL704
3.958
CYS721
3.154
ILE722
3.729
LYS723
3.371
VAL753
3.288
LEU766
3.375
VAL767
4.429
THR768
3.023
GLN769
3.518
|
|||||
| Ligand Name: AMP-PNP | Ligand Info | |||||
| Structure Description | Crystal structure of the catalytically inactive kinase domain of the human epidermal growth factor receptor 3 (HER3) | PDB:3KEX | ||||
| Method | X-ray diffraction | Resolution | 2.80 Å | Mutation | No | [41] |
| PDB Sequence |
KVLARIFKET
688 ELRKLKVLGS698 GVFGTVHKGV708 WIPEGESIKI718 PVCIKVIEDK728 SGRQSFQAVT 738 DHMLAIGSLD748 HAHIVRLLGL758 CPGSSLQLVT768 QYLPLGSLLD778 HVRQHRGALG 788 PQLLLNWGVQ798 IAKGMYYLEE808 HGMVHRNLAA818 RNVLLKSPSQ828 VQVADFGVAD 838 LLPPDDKQLL848 TPIKWMALES863 IHFGKYTHQS873 DVWSYGVTVW883 ELMTFGAEPY 893 AGLRLAEVPD903 LLEKGERLAQ913 PQICTIDVYM923 VMVKCWMIDE933 NIRPTFKELA 943 NEFTRMARDP953 PRYLVIKRES963 GPGIAPGPEP973 HG
|
|||||
|
|
LEU696
3.380
GLY697
3.065
SER698
2.898
GLY699
2.888
VAL700
3.035
PHE701
4.817
GLY702
3.614
VAL704
3.085
CYS721
3.563
LYS723
3.484
VAL753
4.334
THR768
3.897
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |
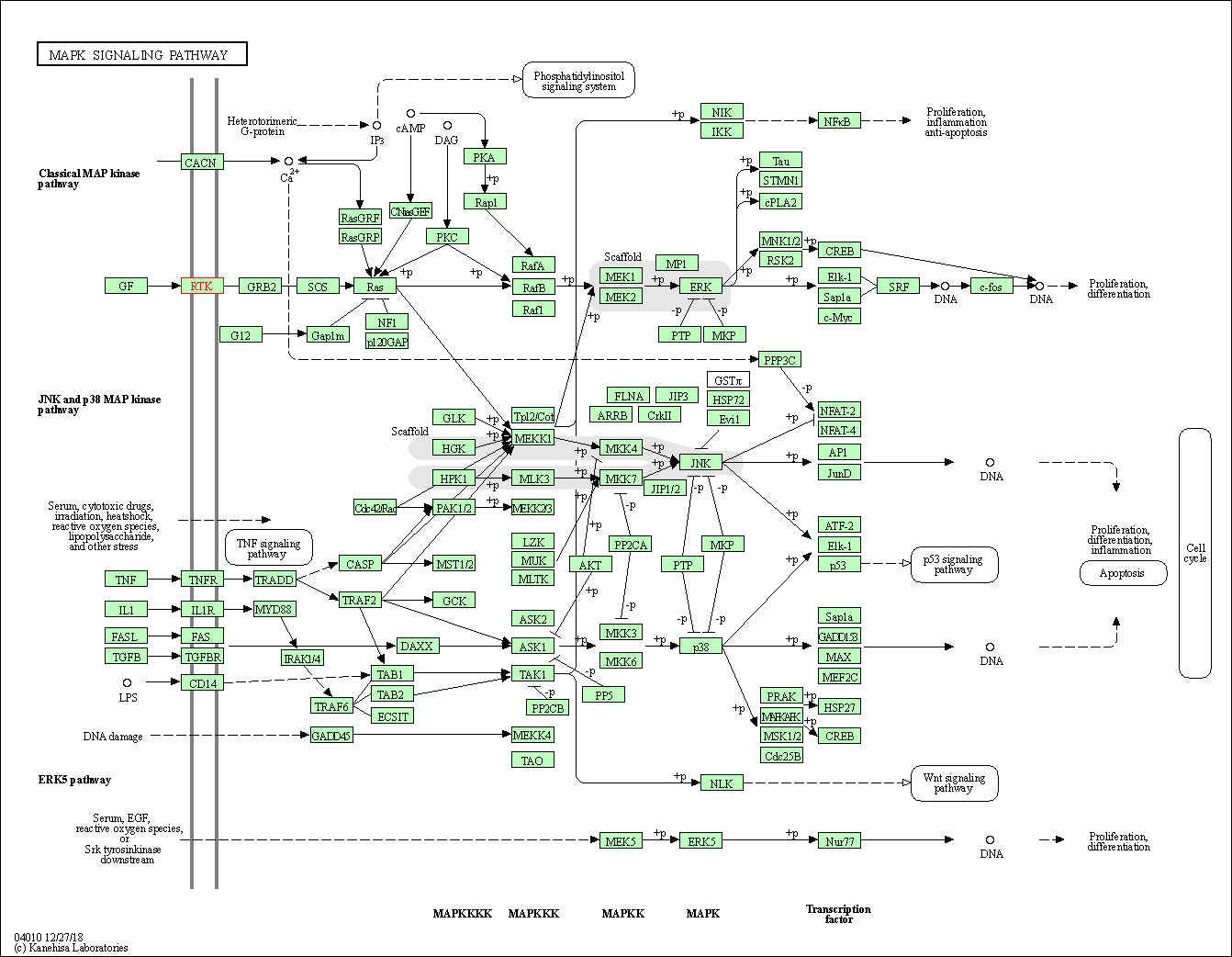
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| ErbB signaling pathway | hsa04012 | Affiliated Target |
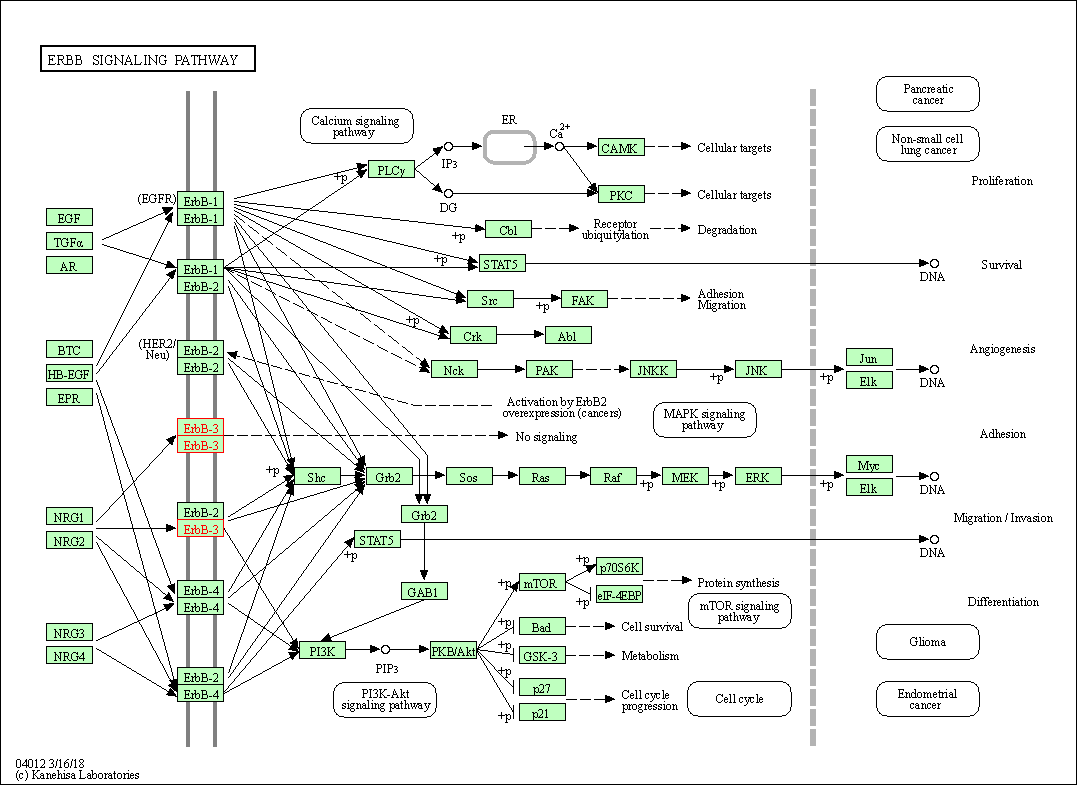
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Calcium signaling pathway | hsa04020 | Affiliated Target |
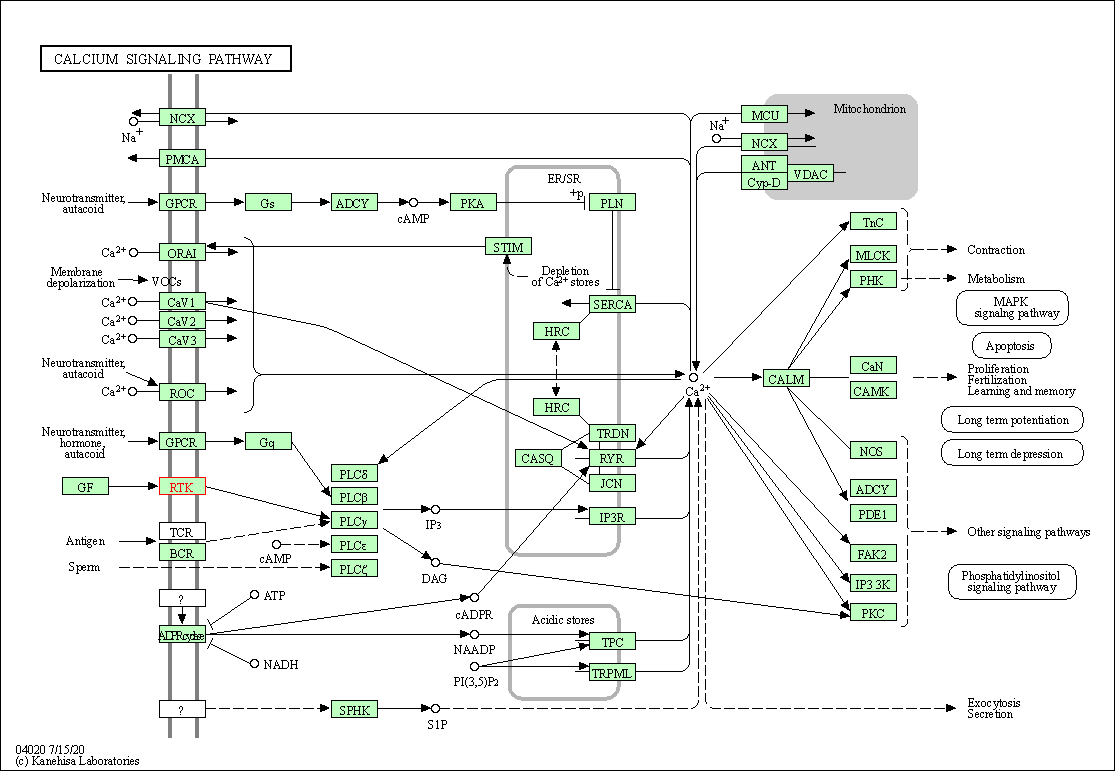
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |
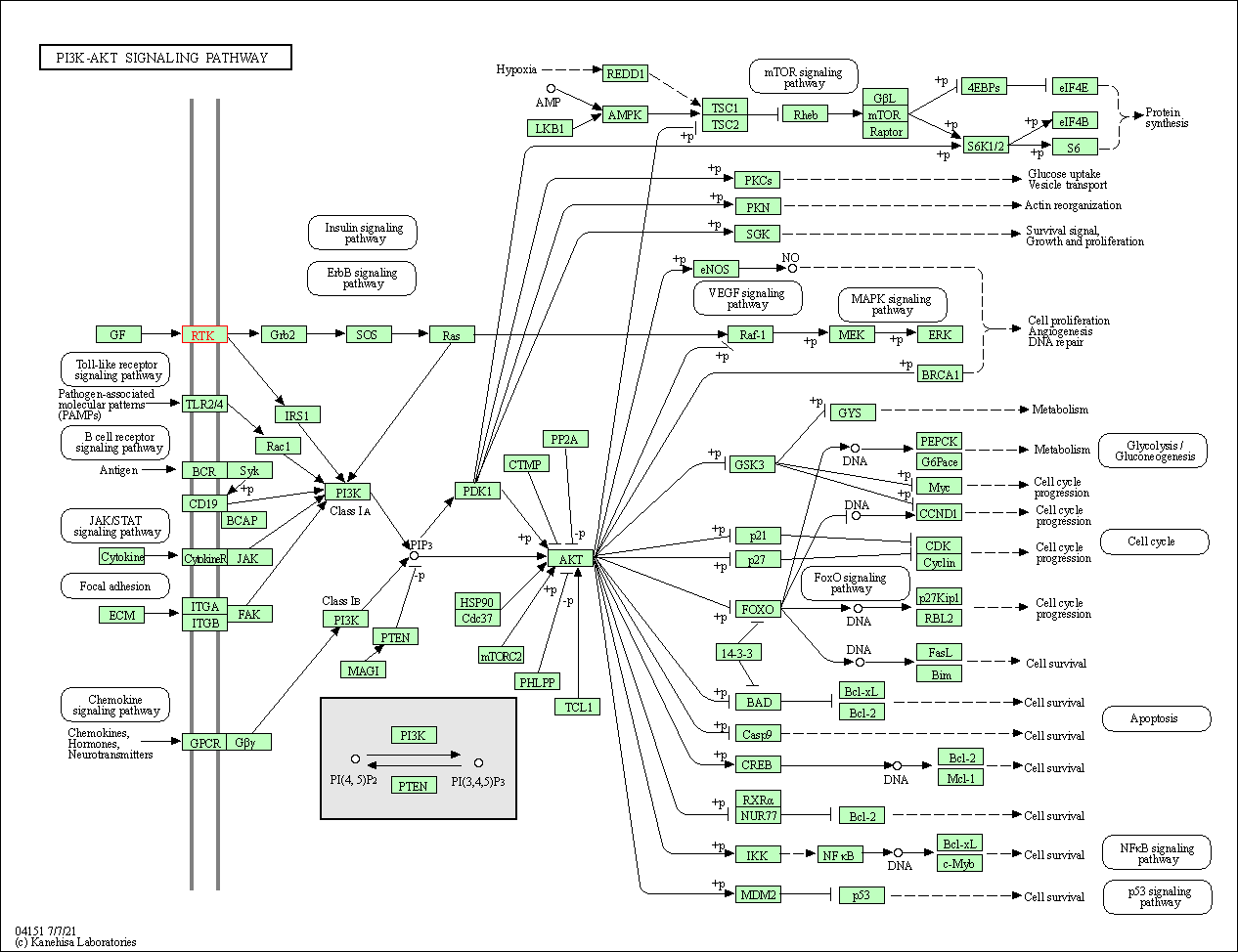
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Degree | 27 | Degree centrality | 2.90E-03 | Betweenness centrality | 1.08E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.50E-01 | Radiality | 1.43E+01 | Clustering coefficient | 3.33E-01 |
| Neighborhood connectivity | 5.21E+01 | Topological coefficient | 8.34E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | ErbB signaling pathway | |||||
| 2 | Calcium signaling pathway | |||||
| 3 | Endocytosis | |||||
| 4 | Proteoglycans in cancer | |||||
| 5 | MicroRNAs in cancer | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | TNFalpha Signaling Pathway | |||||
| Panther Pathway | [+] 2 Panther Pathways | + | ||||
| 1 | Cadherin signaling pathway | |||||
| 2 | EGF receptor signaling pathway | |||||
| PID Pathway | [+] 3 PID Pathways | + | ||||
| 1 | ErbB2/ErbB3 signaling events | |||||
| 2 | ErbB receptor signaling network | |||||
| 3 | a6b1 and a6b4 Integrin signaling | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | ErbB Signaling Pathway | |||||
| 2 | Apoptosis-related network due to altered Notch3 in ovarian cancer | |||||
| 3 | Signaling Pathways in Glioblastoma | |||||
| 4 | miR-targeted genes in muscle cell - TarBase | |||||
| 5 | Heart Development | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Molecular pharmacodynamics of PM02734 (elisidepsin) as single agent and in combination with erlotinib; synergistic activity in human non-small cell lung cancer cell lines and xenograft models. Eur J Cancer. 2009 Jul;45(10):1855-64. | |||||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 3 | First-in-human, phase I study of elisidepsin (PM02734) administered as a 30-min or as a 3-hour intravenous infusion every three weeks in patients with advanced solid tumors. Invest New Drugs. 2015 Aug;33(4):901-10. | |||||
| REF 4 | ClinicalTrials.gov (NCT01577173) A Study of MEHD7945A Versus Cetuximab in Patients With Recurrent/Metastatic Squamous Cell Carcinoma of The Head And Neck | |||||
| REF 5 | ClinicalTrials.gov (NCT01652482) A Study of MEHD7945A + FOLFIRI Versus Cetuximab + FOLFIRI in Second Line in Patients With KRAS Wild-Type Metastatic Colorectal Cancer. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT01447706) A Study of MM-121 With Paclitaxel in Platinum Resistant/ Refractory Advanced Ovarian Cancers. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT02399137) A Phase 2 Study of MM-141 Plus Nab-paclitaxel and Gemcitabine in Front-line Metastatic Pancreatic Cancer. U.S. National Institutes of Health. | |||||
| REF 8 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 9 | ClinicalTrials.gov (NCT02912949) A Phase I/II Study of MCLA-128, a Full Length IgG1 Bispecific Antibody Targeting HER2 and HER3, in Patients With Solid Tumors (eNRGy). U.S.National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT01211483) Study of Erlotinib With or Without Investigational Drug (U3-1287) in Subjects With Advanced Non-small Cell Lung Cancer. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT02143622) Study of Efficacy and Safety of LJM716 and Cetuximab in Head and Neck Squamous Cell Carcinoma Patients. U.S. National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT03321981) MCLA-128 With Trastuzumab/Chemotherapy in HER2+ and With Endocrine Therapy in ER+ and Low HER2 Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 13 | Clinical pipeline report, company report or official report of Genentech (2011). | |||||
| REF 14 | ClinicalTrials.gov (NCT01603979) A Phase 1 Dose Escalation Study of AV-203, an ERBB3 Inhibitory Antibody, in Subjects With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 15 | ClinicalTrials.gov (NCT01966445) Dose Escalation Study to Investigate the Safety, Pharmacokinetics, and Pharmacodynamics of GSK2849330 in Subjects With Advanced Her3-Positive Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 16 | ClinicalTrials.gov (NCT02014909) A Phase 1 Study to Evaluate the Safety and Pharmacokinetics of KTN3379 in Adult Subjects With Advanced Tumors. U.S. National Institutes of Health. | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035447) | |||||
| REF 18 | ClinicalTrials.gov (NCT01957280) Study to Assess Pharmacokinetics, Immunogenicity and Safety/Tolerability of Patritumab (U3-1287). U.S. National Institutes of Health. | |||||
| REF 19 | Clinical pipeline report, company report or official report of Zensun. | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800036915) | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035594) | |||||
| REF 22 | ClinicalTrials.gov (NCT04603287) A Study of SI-B001, an EGFR/HER3 Bispecific Antibody, in Locally Advanced or Metastatic Epithelial Tumors. U.S. National Institutes of Health. | |||||
| REF 23 | Company report (Biooncology) | |||||
| REF 24 | Bispecific antibodies rise again. Nat Rev Drug Discov. 2014 Nov;13(11):799-801. | |||||
| REF 25 | Company report (Merrimack) | |||||
| REF 26 | MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol Cancer Ther. 2014 Feb;13(2):410-25. | |||||
| REF 27 | Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements. Cancer Discov. 2022 May 2;12(5):1233-1247. | |||||
| REF 28 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1798). | |||||
| REF 29 | An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin. Cancer Res. 2013 Oct 1;73(19):6024-35. | |||||
| REF 30 | Clinical pipeline report, company report or official report of Merus. | |||||
| REF 31 | Neuregulin 1 expression is a predictive biomarker for response to AV-203, an ERBB3 inhibitory antibody, in human tumor models. Clin Cancer Res. 2015 Mar 1;21(5):1106-14. | |||||
| REF 32 | National Cancer Institute Drug Dictionary (drug id 754219). | |||||
| REF 33 | Therapeutic target database update 2012: a resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012 Jan;40(Database issue):D1128-36. | |||||
| REF 34 | A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res. 2015; 7(4): 733-750. | |||||
| REF 35 | Phase 1 and dose-finding study of patritumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014 Mar;73(3):511-6. | |||||
| REF 36 | Clinical pipeline report, company report or official report of Zensun. | |||||
| REF 37 | J Clin Oncol 32:5s, 2014 (suppl; abstr 2516). | |||||
| REF 38 | RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res. 2013 Aug 15;73(16):5183-94. | |||||
| REF 39 | Clinical pipeline report, company report or official report of SystImmune. | |||||
| REF 40 | Targetable HER3 functions driving tumorigenic signaling in HER2-amplified cancers. Cell Rep. 2022 Feb 1;38(5):110291. | |||||
| REF 41 | Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc Natl Acad Sci U S A. 2009 Dec 22;106(51):21608-13. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

