Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T38529
(Former ID: TTDS00168)
|
|||||
| Target Name |
Prostaglandin E2 receptor EP2 (PTGER2)
|
|||||
| Synonyms |
Prostanoid EP2 receptor; Prostaglandin E2 receptor EP2 subtype; PGE2 receptor EP2 subtype; PGE receptor, EP2 subtype; PGE receptor EP2 subtype; EP2 receptor
Click to Show/Hide
|
|||||
| Gene Name |
PTGER2
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 7 Target-related Diseases | + | ||||
| 1 | Abortion [ICD-11: JA00] | |||||
| 2 | Diabetic foot ulcer [ICD-11: BD54] | |||||
| 3 | Glaucoma [ICD-11: 9C61] | |||||
| 4 | Pulmonary hypertension [ICD-11: BB01] | |||||
| 5 | Sexual dysfunction [ICD-11: HA00-HA01] | |||||
| 6 | Transplant rejection [ICD-11: NE84] | |||||
| 7 | Coronary atherosclerosis [ICD-11: BA80] | |||||
| Function |
The activity of this receptor is mediated by G(s) proteins that stimulate adenylate cyclase. The subsequent raise in intracellular cAMP is responsible for the relaxing effect of this receptor on smooth muscle. Receptor for prostaglandin E2 (PGE2).
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
MGNASNDSQSEDCETRQWLPPGESPAISSVMFSAGVLGNLIALALLARRWRGDVGCSAGR
RSSLSLFHVLVTELVFTDLLGTCLISPVVLASYARNQTLVALAPESRACTYFAFAMTFFS LATMLMLFAMALERYLSIGHPYFYQRRVSRSGGLAVLPVIYAVSLLFCSLPLLDYGQYVQ YCPGTWCFIRHGRTAYLQLYATLLLLLIVSVLACNFSVILNLIRMHRRSRRSRCGPSLGS GRGGPGARRRGERVSMAEETDHLILLAIMTITFAVCSLPFTIFAYMNETSSRKEKWDLQA LRFLSINSIIDPWVFAILRPPVLRLMRSVLCCRISLRTQDATQTSCSTQSDASKQADL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T46TBY | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 6 Approved Drugs | + | ||||
| 1 | Alprostadil | Drug Info | Approved | Erectile dysfunction | [2], [3] | |
| 2 | Dinoprostone | Drug Info | Approved | Medical abortion | [4], [5] | |
| 3 | Iloprost | Drug Info | Approved | Pulmonary arterial hypertension | [6], [7], [8] | |
| 4 | Mycophenolate mofetil | Drug Info | Approved | Organ transplant rejection | [9], [10] | |
| 5 | Omidenepag isopropyl | Drug Info | Approved | Glaucoma/ocular hypertension | [11] | |
| 6 | LAROPIPRANT | Drug Info | Phase 4 | Coronary heart disease | [12] | |
| Clinical Trial Drug(s) | [+] 6 Clinical Trial Drugs | + | ||||
| 1 | 16,16-dimethyl-PGE2 | Drug Info | Phase 2 | Stem cell engraftment | [13] | |
| 2 | CP-533536 | Drug Info | Phase 2 | Osteoporosis | [14], [15] | |
| 3 | Taprenepag | Drug Info | Phase 2 | Glaucoma/ocular hypertension | [16], [17] | |
| 4 | PF-4418948 | Drug Info | Phase 1 | Endometriosis | [18], [19] | |
| 5 | TPST-1495 | Drug Info | Phase 1 | Solid tumour/cancer | [20] | |
| 6 | PGF2alpha | Drug Info | Clinical trial | Solid tumour/cancer | [21] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | ONO-8815Ly | Drug Info | Terminated | Miscarriage | [22], [23] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Agonist | [+] 21 Agonist drugs | + | ||||
| 1 | Alprostadil | Drug Info | [24] | |||
| 2 | Iloprost | Drug Info | [1] | |||
| 3 | Omidenepag isopropyl | Drug Info | [28] | |||
| 4 | 16,16-dimethyl-PGE2 | Drug Info | [30] | |||
| 5 | CP-533536 | Drug Info | [15], [31] | |||
| 6 | Taprenepag | Drug Info | [32] | |||
| 7 | PGF2alpha | Drug Info | [35] | |||
| 8 | ONO-8815Ly | Drug Info | [37] | |||
| 9 | 11-deoxy-PGE1 | Drug Info | [30] | |||
| 10 | 17-phenyl-omega-trinor-PGE2 | Drug Info | [38] | |||
| 11 | 19(R)-OH-PGE2 | Drug Info | [38] | |||
| 12 | AH13205 | Drug Info | [30] | |||
| 13 | butaprost (free acid form) | Drug Info | [35] | |||
| 14 | carbacyclin | Drug Info | [40] | |||
| 15 | cicaprost | Drug Info | [30] | |||
| 16 | isocarbacyclin | Drug Info | [30] | |||
| 17 | M&B 28767 | Drug Info | [38] | |||
| 18 | ONO-AE-248 | Drug Info | [41] | |||
| 19 | ONO-AE1-329 | Drug Info | [41] | |||
| 20 | PGD2 | Drug Info | [35] | |||
| 21 | U46619 | Drug Info | [38] | |||
| Antagonist | [+] 23 Antagonist drugs | + | ||||
| 1 | Dinoprostone | Drug Info | [25], [26] | |||
| 2 | PF-4418948 | Drug Info | [25], [33] | |||
| 3 | TPST-1495 | Drug Info | [34] | |||
| 4 | BUTAPROST | Drug Info | [25], [36] | |||
| 5 | PMID25772215-Compound-EP20082149552C9 | Drug Info | [25] | |||
| 6 | PMID25772215-Compound-EP2149552diaminopyrimidines | Drug Info | [25] | |||
| 7 | PMID25772215-Compound-US02014179750M1 | Drug Info | [25] | |||
| 8 | PMID25772215-Compound-US02014179750M2 | Drug Info | [25] | |||
| 9 | PMID25772215-Compound-US02014179750M3 | Drug Info | [25] | |||
| 10 | PMID25772215-Compound-US02014179750TG6-10-1 | Drug Info | [25] | |||
| 11 | PMID25772215-Compound-US02014179750TG6-129 | Drug Info | [25] | |||
| 12 | PMID25772215-Compound-US02014179750TG7-112-2 | Drug Info | [25] | |||
| 13 | PMID25772215-Compound-US02014179750TG7-170 | Drug Info | [25] | |||
| 14 | PMID25772215-Compound-US02014179750TG7-74 | Drug Info | [25] | |||
| 15 | PMID25772215-Compound-US02014179750TG7-76 | Drug Info | [25] | |||
| 16 | PMID25772215-Compound-US02014179750TG8-15 | Drug Info | [25] | |||
| 17 | PMID25772215-Compound-WO2012177618M4 | Drug Info | [25] | |||
| 18 | PMID25772215-Compound-WO2012177618M5 | Drug Info | [25] | |||
| 19 | PMID25772215-Compound-WO2012177618M6 | Drug Info | [25] | |||
| 20 | AH6809 | Drug Info | [40] | |||
| 21 | PF-00212062 | Drug Info | [27] | |||
| 22 | TG4-155 | Drug Info | [43] | |||
| 23 | TG7-171 | Drug Info | [44] | |||
| Modulator | [+] 2 Modulator drugs | + | ||||
| 1 | Mycophenolate mofetil | Drug Info | [27] | |||
| 2 | R-65 | Drug Info | [27] | |||
| Inhibitor | [+] 5 Inhibitor drugs | + | ||||
| 1 | LAROPIPRANT | Drug Info | [29] | |||
| 2 | 3-(2-((E)-3-phenylprop-1-enyl)phenyl)acrylic acid | Drug Info | [39] | |||
| 3 | 3-(2-(4-methoxycinnamyl)phenyl)acrylic acid | Drug Info | [39] | |||
| 4 | 3-(2-(naphthalen-2-ylmethyl)phenyl)acrylic acid | Drug Info | [39] | |||
| 5 | 3-(2-cinnamylphenyl)acrylic acid | Drug Info | [39] | |||
| Modulator (allosteric modulator) | [+] 1 Modulator (allosteric modulator) drugs | + | ||||
| 1 | PMID20080612C1 | Drug Info | [42] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Dinoprostone | Ligand Info | |||||
| Structure Description | Cryo-EM structure of the PGE2-bound EP2-Gs complex | PDB:7CX2 | ||||
| Method | Electron microscopy | Resolution | 2.80 Å | Mutation | Yes | [45] |
| PDB Sequence |
ESPAISSVMF
32 SAGVLGNLIA42 LALLARSLFH68 VLVTELVFTD78 LLGTCLISPV88 VLASYARNQT 98 LVALAPESRA108 CTYFAFAMTF118 FSLATMLMLF128 AMALERYLSI138 GHPYFYQRRV 148 SRSGGLAVLP158 VIYAVSLLFC168 SLPLLDYGQY178 VQYCPGTWCF188 IRHGRTAYLQ 198 LYATLLLLLI208 VSVLACNFSV218 ILNLIRMHRR228 SRAEETDHLI264 LLAIMTITFA 274 VCSLPFTIFA284 YMNETSSRKE294 KWDLQALRFL304 SINSIIDPWV314 FAILRPPVLR 324 LMRSVL
|
|||||
|
|
GLU23
4.394
SER28
2.624
MET31
3.604
PHE32
4.353
GLY81
4.424
THR82
3.445
ILE85
3.822
SER86
2.665
VAL89
3.246
TYR93
3.181
PHE112
4.831
MET116
3.217
PHE119
4.567
|
|||||
| Ligand Name: Taprenepag | Ligand Info | |||||
| Structure Description | Cryo-EM structure of the Taprenepag-bound EP2-Gs complex | PDB:7CX3 | ||||
| Method | Electron microscopy | Resolution | 2.80 Å | Mutation | Yes | [45] |
| PDB Sequence |
ESPAISSVMF
32 SAGVLGNLIA42 LALLARSLFH68 VLVTELVFTD78 LLGTCLISPV88 VLASYARNQT 98 LVALAPESRA108 CTYFAFAMTF118 FSLATMLMLF128 AMALERYLSI138 GHPYFYQRRV 148 SRSGGLAVLP158 VIYAVSLLFC168 SLPLLDYGQY178 VQYCPGTWCF188 IRHGRTAYLQ 198 LYATLLLLLI208 VSVLACNFSV218 ILNLIRMHRR228 SAEETDHLIL265 LAIMTITFAV 275 CSLPFTIFAY285 MNETSSRKEK295 WDLQALRFLS305 INSIIDPWVF315 AILRPPVLRL 325 MRSVL
|
|||||
|
|
SER28
2.967
MET31
3.389
PHE32
3.863
GLY35
4.999
THR77
4.297
ASP78
3.712
GLY81
3.702
THR82
2.796
ILE85
3.479
SER86
2.649
VAL89
3.227
TYR93
3.368
PHE112
4.776
MET116
3.455
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Similarity Proteins
Human Pathway Affiliation
|
|
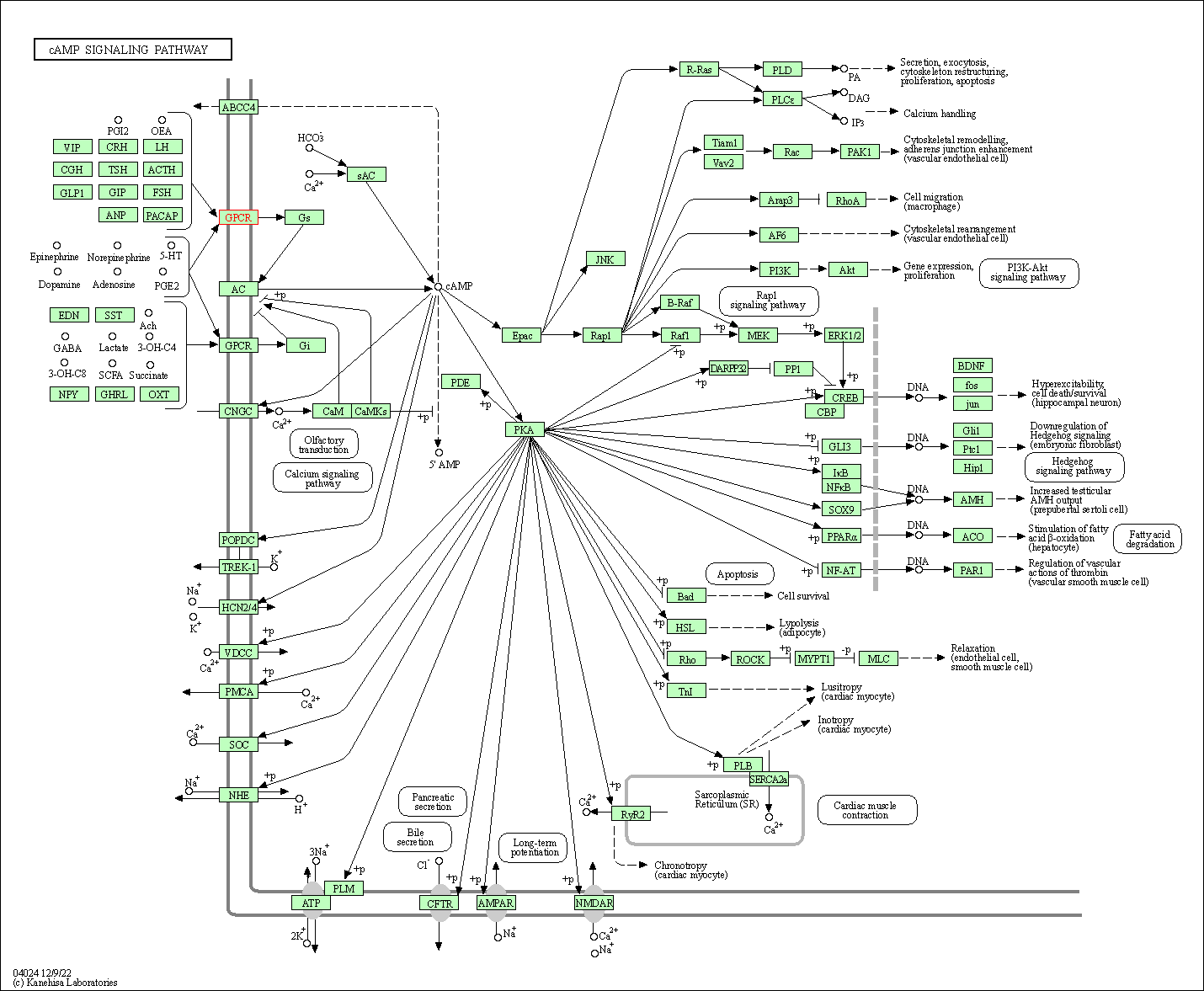
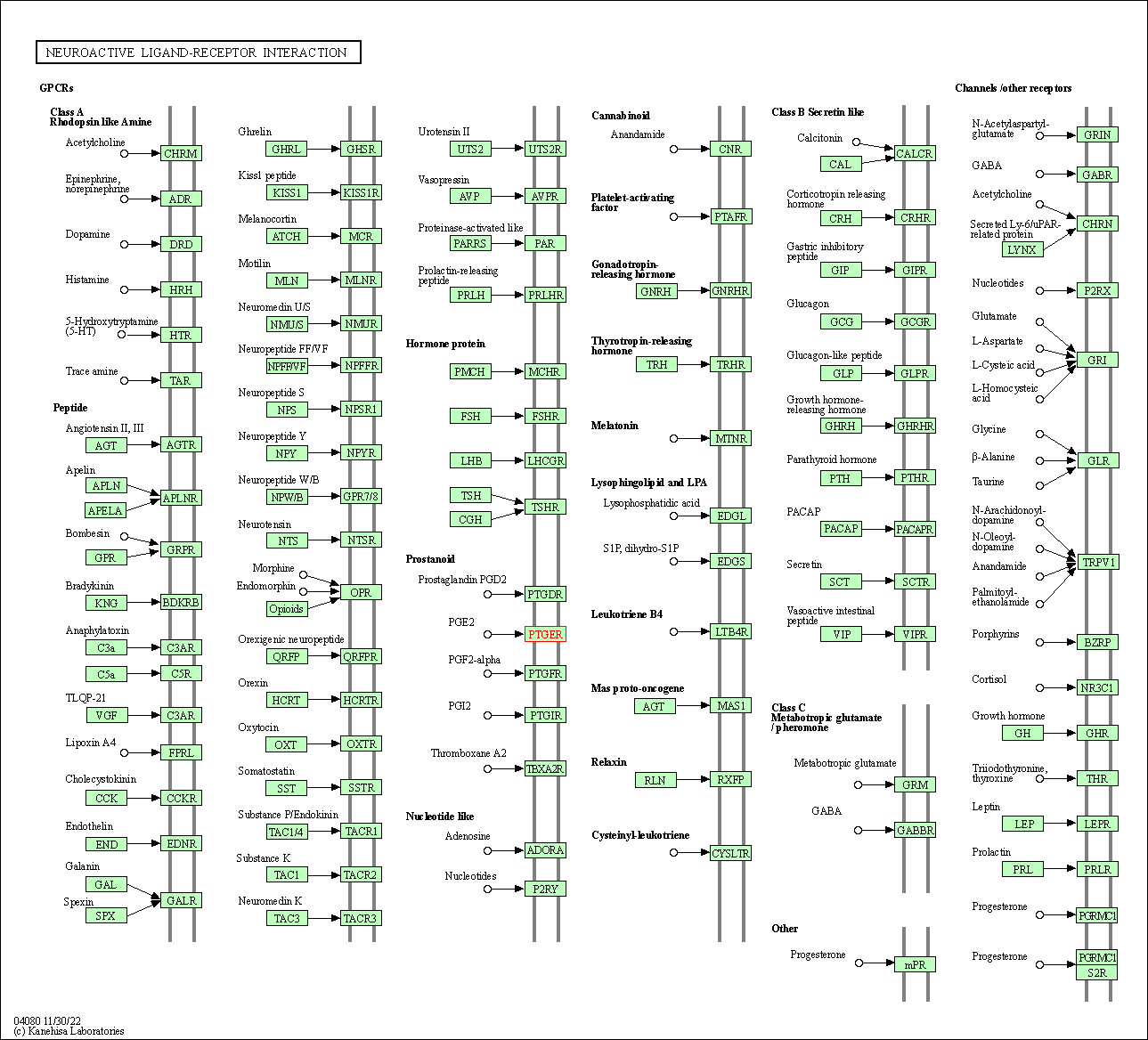
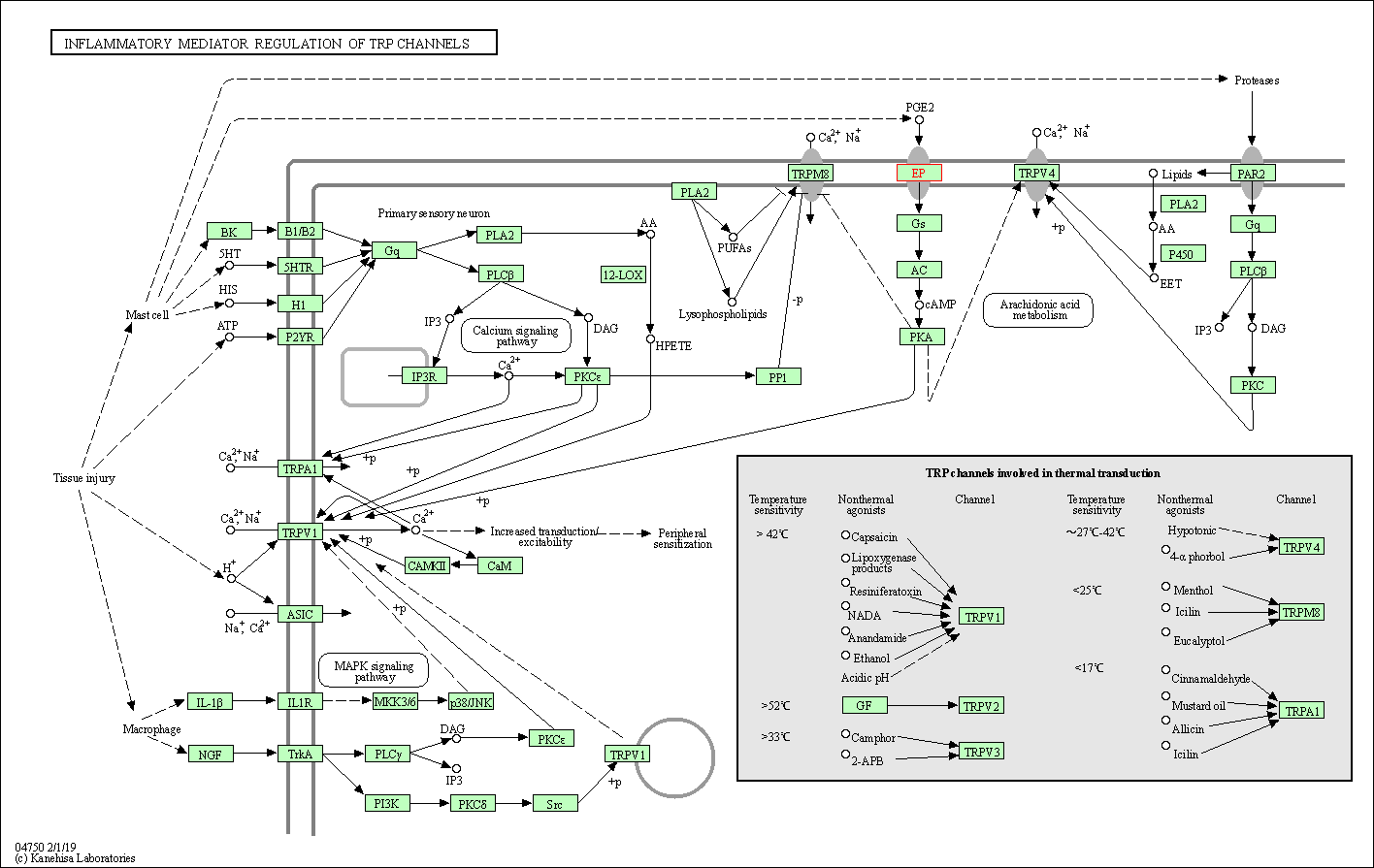
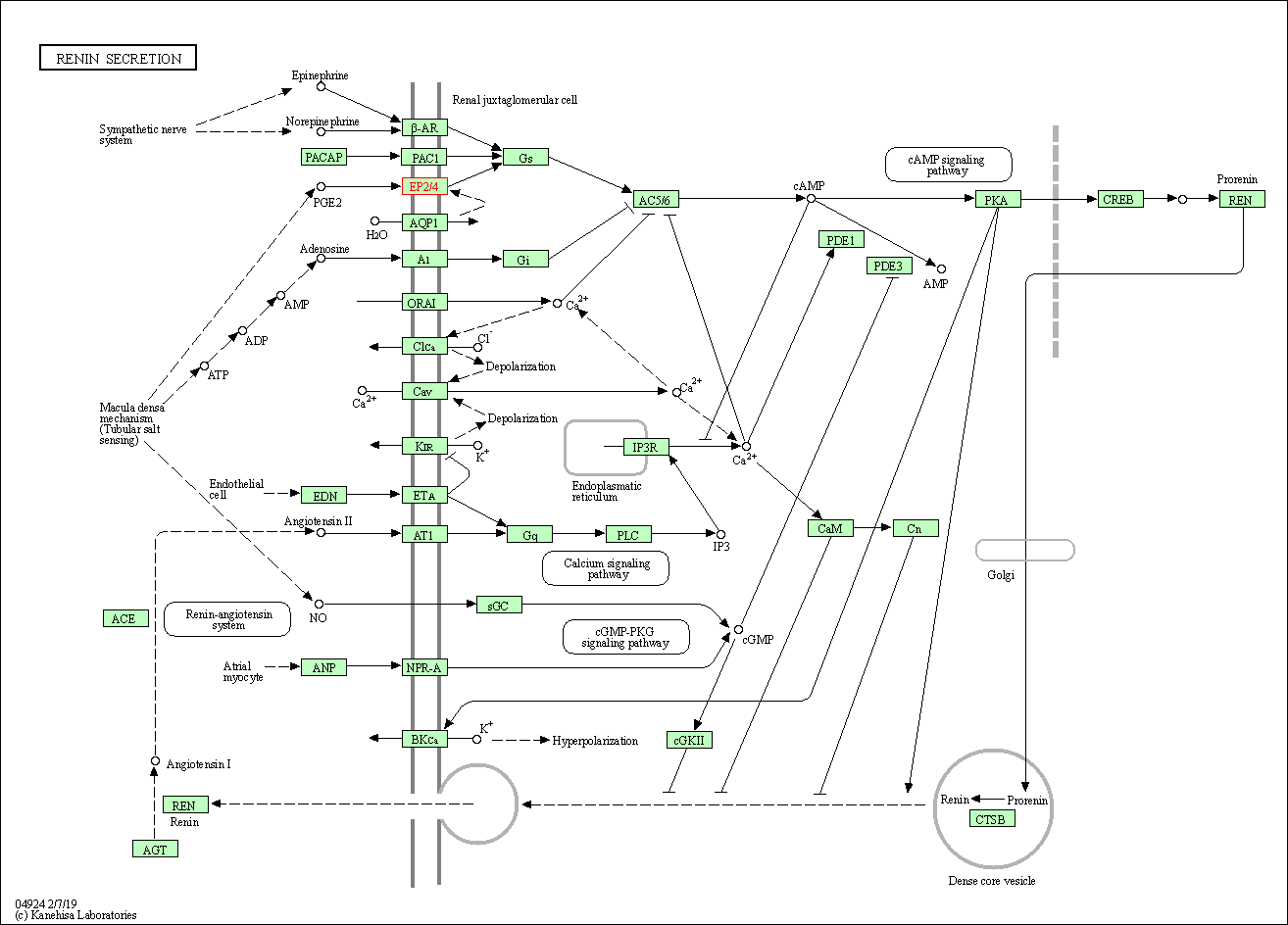
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| cAMP signaling pathway | hsa04024 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Inflammatory mediator regulation of TRP channels | hsa04750 | Affiliated Target |

|
| Class: Organismal Systems => Sensory system | Pathway Hierarchy | ||
| Renin secretion | hsa04924 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | cAMP signaling pathway | |||||
| 2 | Neuroactive ligand-receptor interaction | |||||
| 3 | Inflammatory mediator regulation of TRP channels | |||||
| 4 | Renin secretion | |||||
| 5 | Pathways in cancer | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Prostanoid ligand receptors | |||||
| 2 | G alpha (s) signalling events | |||||
| WikiPathways | [+] 6 WikiPathways | + | ||||
| 1 | Prostaglandin Synthesis and Regulation | |||||
| 2 | GPCRs, Class A Rhodopsin-like | |||||
| 3 | Ovarian Infertility Genes | |||||
| 4 | Small Ligand GPCRs | |||||
| 5 | GPCR ligand binding | |||||
| 6 | GPCR downstream signaling | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Exploration of prostanoid receptor subtype regulating estradiol and prostaglandin E2 induction of spinophilin in developing preoptic area neurons. Neuroscience. 2007 May 25;146(3):1117-27. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1882). | |||||
| REF 3 | Emerging therapies for treatment of acute lung injury and acute respiratory distress syndrome. Expert Opin Emerg Drugs. 2007 Sep;12(3):461-77. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1916). | |||||
| REF 5 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 020411. | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1966). | |||||
| REF 7 | 2004 approvals: the demise of the blockbuster. Nat Rev Drug Discov. 2005 Feb;4(2):93-4. | |||||
| REF 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 9 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6831). | |||||
| REF 10 | Emerging drugs for idiopathic thrombocytopenic purpura in adults. Expert Opin Emerg Drugs. 2008 Jun;13(2):237-54. | |||||
| REF 11 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 215092. | |||||
| REF 12 | ClinicalTrials.gov (NCT01126073) A Double Blind, Randomized Study to Compare Influence of Niacin/Laropiprant on Functional and Morphological Characteristics of Arterial Wall and Parameters of Inflammation in Subjects With Coronary Heart Disease Already Treated With a Statin in Miran Sebestjen, University Medical Centre Ljubljana. | |||||
| REF 13 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1929). | |||||
| REF 15 | Discovery of CP-533536: an EP2 receptor selective prostaglandin E2 (PGE2) agonist that induces local bone formation. Bioorg Med Chem Lett. 2009 Apr 1;19(7):2075-8. | |||||
| REF 16 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5816). | |||||
| REF 17 | ClinicalTrials.gov (NCT00572455) Safety and Efficacy of PF-04217329 in Patients With Glaucoma or Elevated Eye Pressure.. U.S. National Institutes of Health. | |||||
| REF 18 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5817). | |||||
| REF 19 | ClinicalTrials.gov (NCT01002963) A Study To Investigate The Safety And Toleration Of A Single Dose Of PF-04418948 In Healthy Volunteers. U.S. National Institutes of Health. | |||||
| REF 20 | ClinicalTrials.gov (NCT04344795) Phase 1a/1b Study of TPST-1495 in Subjects With Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 21 | Stereocontrolled organocatalytic synthesis of prostaglandin PGF2alpha in seven steps. Nature. 2012 Sep 13;489(7415):278-81. | |||||
| REF 22 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1932). | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012532) | |||||
| REF 24 | Emerging drugs for diabetic foot ulcers. Expert Opin Emerg Drugs. 2006 Nov;11(4):709-24. | |||||
| REF 25 | Evaluation of WO 2012/177618 A1 and US-2014/0179750 A1: novel small molecule antagonists of prostaglandin-E2 receptor EP2.Expert Opin Ther Pat. 2015 Jul;25(7):837-44. | |||||
| REF 26 | Concurrent dinoprostone and oxytocin for labor induction in term premature rupture of membranes: a randomized controlled trial. Obstet Gynecol. 2009 May;113(5):1059-65. | |||||
| REF 27 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 341). | |||||
| REF 28 | Clinical pipeline report, company report or official report of Santen Pharmaceutical. | |||||
| REF 29 | Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydroc... J Med Chem. 2007 Feb 22;50(4):794-806. | |||||
| REF 30 | Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997 Sep;122(2):217-24. | |||||
| REF 31 | Pfizer. Product Development Pipeline. March 31 2009. | |||||
| REF 32 | A phase 2, randomized, dose-response trial of taprenepag isopropyl (PF-04217329) versus latanoprost 0.005% in open-angle glaucoma and ocular hypertension. Curr Eye Res. 2011 Sep;36(9):809-17. | |||||
| REF 33 | Clinical pipeline report, company report or official report of axonmedchem. | |||||
| REF 34 | Clinical pipeline report, company report or official report of Tempest Therapeutics. | |||||
| REF 35 | Importance of the extracellular domain for prostaglandin EP(2) receptor function. Mol Pharmacol. 1999 Sep;56(3):545-51. | |||||
| REF 36 | Synthesis and evaluation of a gamma-lactam as a highly selective EP2 and EP4 receptor agonist. Bioorg Med Chem Lett. 2008 Jan 15;18(2):821-4. | |||||
| REF 37 | Prostaglandin E2 receptor type 2-selective agonist prevents the degeneration of articular cartilage in rabbit knees with traumatic instability. Arthritis Res Ther. 2011;13(5):R146. | |||||
| REF 38 | Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol. 1997 Dec 11;340(2-3):227-41. | |||||
| REF 39 | Comparison between two classes of selective EP(3) antagonists and their biological activities. Bioorg Med Chem Lett. 2006 Nov 1;16(21):5639-42. | |||||
| REF 40 | The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000 Jan 17;1483(2):285-93. | |||||
| REF 41 | The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000 Apr;141(4):1554-9. | |||||
| REF 42 | Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc Natl Acad Sci U S A. 2010 Feb 2;107(5):2307-12. | |||||
| REF 43 | Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci U S A. 2012 Feb 21;109(8):3149-54. | |||||
| REF 44 | Development of second generation EP2 antagonists with high selectivity. Eur J Med Chem. 2014 Jul 23;82:521-35. | |||||
| REF 45 | Ligand recognition, unconventional activation, and G protein coupling of the prostaglandin E(2) receptor EP2 subtype. Sci Adv. 2021 Apr 2;7(14):eabf1268. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

