Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0WL5V
|
|||
| Former ID |
DNC007125
|
|||
| Drug Name |
LAROPIPRANT
|
|||
| Synonyms |
Laropiprant; 571170-77-9; MK 0524; Cardaptive; MK-0524; UNII-G7N11T8O78; CHEMBL426559; G7N11T8O78; 2-[(3R)-4-[(4-chlorophenyl)methyl]-7-fluoro-5-methylsulfonyl-2,3-dihydro-1H-cyclopenta[b]indol-3-yl]acetic acid; 2-[(3R)-4-[(4-chlorophenyl)methyl]-7-fluoro-5-methanesulfonyl-1H,2H,3H,4H-cyclopenta[b]indol-3-yl]acetic acid; Tedaptive; Laropiprant [USAN:INN:BAN]; [14C]-Laropiprant; Laropiprant/MK-0524; Laropiprant (INN/USAN); Laropiprant (MK-0524); SCHEMBL991107; AMOT0189; GTPL3356; KS-00000XIE; CTK8F0660; MK-0524B
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronary heart disease [ICD-11: BA80.Z] | Phase 4 | [1] | |
| Arteriosclerosis [ICD-11: BD40; ICD-10: I70] | Discontinued in Phase 3 | [2] | ||
| Structure |
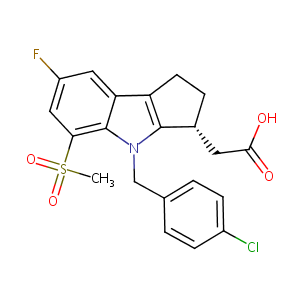 |
Download2D MOL |
||
| Formula |
C21H19ClFNO4S
|
|||
| Canonical SMILES |
CS(=O)(=O)C1=CC(=CC2=C1N(C3=C2CCC3CC(=O)O)CC4=CC=C(C=C4)Cl)F
|
|||
| InChI |
1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m1/s1
|
|||
| InChIKey |
NXFFJDQHYLNEJK-CYBMUJFWSA-N
|
|||
| CAS Number |
CAS 571170-77-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14832522, 16319858, 24159188, 45289906, 79316133, 96025623, 103506442, 104083674, 125240979, 126671694, 126731356, 134339127, 134344330, 135195695, 136367874, 137185589, 139755590, 144116232, 152255074, 152258799, 152344287, 160647650, 162012049, 162205095, 163125572, 164023436, 172087022, 175266118, 178100382, 186014491, 204366771, 223398991, 223556714, 223674460, 225130865, 227246809, 247021101, 249816641, 251971100, 252451672
|
|||
| ChEBI ID |
CHEBI:135942
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01126073) A Double Blind, Randomized Study to Compare Influence of Niacin/Laropiprant on Functional and Morphological Characteristics of Arterial Wall and Parameters of Inflammation in Subjects With Coronary Heart Disease Already Treated With a Statin in Miran Sebestjen, University Medical Centre Ljubljana. | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023729) | |||
| REF 3 | A novel c-Met inhibitor, MK8033, synergizes with carboplatin plus paclitaxel to inhibit ovarian cancer cell growth. Oncol Rep. 2013 May;29(5):2011-8. | |||
| REF 4 | Selective Targeting of CTNBB1-, KRAS- or MYC-Driven Cell Growth by Combinations of Existing Drugs. PLoS One. 2015 May 27;10(5):e0125021. | |||
| REF 5 | Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydroc... J Med Chem. 2007 Feb 22;50(4):794-806. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

