Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T99954
(Former ID: TTDS00170)
|
|||||
| Target Name |
Prostacyclin receptor (PTGIR)
|
|||||
| Synonyms |
Prostanoid IP receptor; Prostaglandin I2 receptor; PRIPR; PGI2 receptor; PGI receptor; IP prostanoid receptor
Click to Show/Hide
|
|||||
| Gene Name |
PTGIR
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Abortion [ICD-11: JA00] | |||||
| 2 | Pulmonary hypertension [ICD-11: BB01] | |||||
| 3 | Coronary atherosclerosis [ICD-11: BA80] | |||||
| Function |
The activity of this receptor is mediated by G(s) proteins which activate adenylate cyclase. Receptor for prostacyclin (prostaglandin I2 or PGI2).
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
MADSCRNLTYVRGSVGPATSTLMFVAGVVGNGLALGILSARRPARPSAFAVLVTGLAATD
LLGTSFLSPAVFVAYARNSSLLGLARGGPALCDAFAFAMTFFGLASMLILFAMAVERCLA LSHPYLYAQLDGPRCARLALPAIYAFCVLFCALPLLGLGQHQQYCPGSWCFLRMRWAQPG GAAFSLAYAGLVALLVAAIFLCNGSVTLSLCRMYRQQKRHQGSLGPRPRTGEDEVDHLIL LALMTVVMAVCSLPLTIRCFTQAVAPDSSSEMGDLLAFRFYAFNPILDPWVFILFRKAVF QRLKLWVCCLCLGPAHGDSQTPLSQLASGRRDPRAPSAPVGKEGSCVPLSAWGEGQVEPL PPTQQSSGSAVGTSSKAEASVACSLC Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 7 Approved Drugs | + | ||||
| 1 | Dinoprost Tromethamine | Drug Info | Approved | Abortion | [2] | |
| 2 | Epoprostenol | Drug Info | Approved | Pulmonary hypertension | [3], [4] | |
| 3 | Misoprostol | Drug Info | Approved | Medical abortion | [5], [6] | |
| 4 | Selexipag | Drug Info | Approved | Pulmonary arterial hypertension | [7], [8] | |
| 5 | Treprostinil | Drug Info | Approved | Pulmonary arterial hypertension | [4], [9] | |
| 6 | Beraprost | Drug Info | Phase 4 | Pulmonary hypertension | [10] | |
| 7 | LAROPIPRANT | Drug Info | Phase 4 | Coronary heart disease | [11] | |
| Clinical Trial Drug(s) | [+] 2 Clinical Trial Drugs | + | ||||
| 1 | esuberaprost | Drug Info | Phase 3 | Gout | [12] | |
| 2 | Ralinepag | Drug Info | Phase 2 | Pulmonary hypertension | [13] | |
| Discontinued Drug(s) | [+] 5 Discontinued Drugs | + | ||||
| 1 | Clinprost | Drug Info | Discontinued in Preregistration | Asthma | [14] | |
| 2 | Ataprost | Drug Info | Discontinued in Phase 3 | Asthma | [15] | |
| 3 | YM533 | Drug Info | Discontinued in Phase 3 | Chronic renal failure | [16] | |
| 4 | OP-2507 | Drug Info | Discontinued in Phase 2 | Hypertension | [17] | |
| 5 | EPTALOPROST | Drug Info | Terminated | Solid tumour/cancer | [18] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Antagonist | [+] 5 Antagonist drugs | + | ||||
| 1 | Dinoprost Tromethamine | Drug Info | [1], [19] | |||
| 2 | Misoprostol | Drug Info | [1], [23], [24] | |||
| 3 | BAY-73-1449 | Drug Info | [38] | |||
| 4 | RO1138452 | Drug Info | [45] | |||
| 5 | RO3244794 | Drug Info | [45] | |||
| Agonist | [+] 15 Agonist drugs | + | ||||
| 1 | Epoprostenol | Drug Info | [20], [21], [22] | |||
| 2 | Selexipag | Drug Info | [25] | |||
| 3 | Treprostinil | Drug Info | [26] | |||
| 4 | Beraprost | Drug Info | [27] | |||
| 5 | esuberaprost | Drug Info | [12] | |||
| 6 | Ralinepag | Drug Info | [29] | |||
| 7 | AFP-07 | Drug Info | [37] | |||
| 8 | BMY 45778 | Drug Info | [39] | |||
| 9 | butaprost (free acid form) | Drug Info | [40] | |||
| 10 | carbacyclin | Drug Info | [40] | |||
| 11 | cicaprost | Drug Info | [41] | |||
| 12 | EP 157 | Drug Info | [42] | |||
| 13 | isocarbacyclin | Drug Info | [41] | |||
| 14 | TEI-9063 | Drug Info | [39] | |||
| 15 | U46619 | Drug Info | [40] | |||
| Inhibitor | [+] 4 Inhibitor drugs | + | ||||
| 1 | LAROPIPRANT | Drug Info | [28] | |||
| 2 | AP-227 | Drug Info | [34] | |||
| 3 | FR-181157 | Drug Info | [43] | |||
| 4 | FR-193262 | Drug Info | [44] | |||
| Modulator | [+] 6 Modulator drugs | + | ||||
| 1 | Clinprost | Drug Info | [30] | |||
| 2 | Ataprost | Drug Info | [31] | |||
| 3 | YM533 | Drug Info | [32] | |||
| 4 | OP-2507 | Drug Info | [33] | |||
| 5 | EPTALOPROST | Drug Info | [35], [36] | |||
| 6 | Prostacyclin analog | Drug Info | [32] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
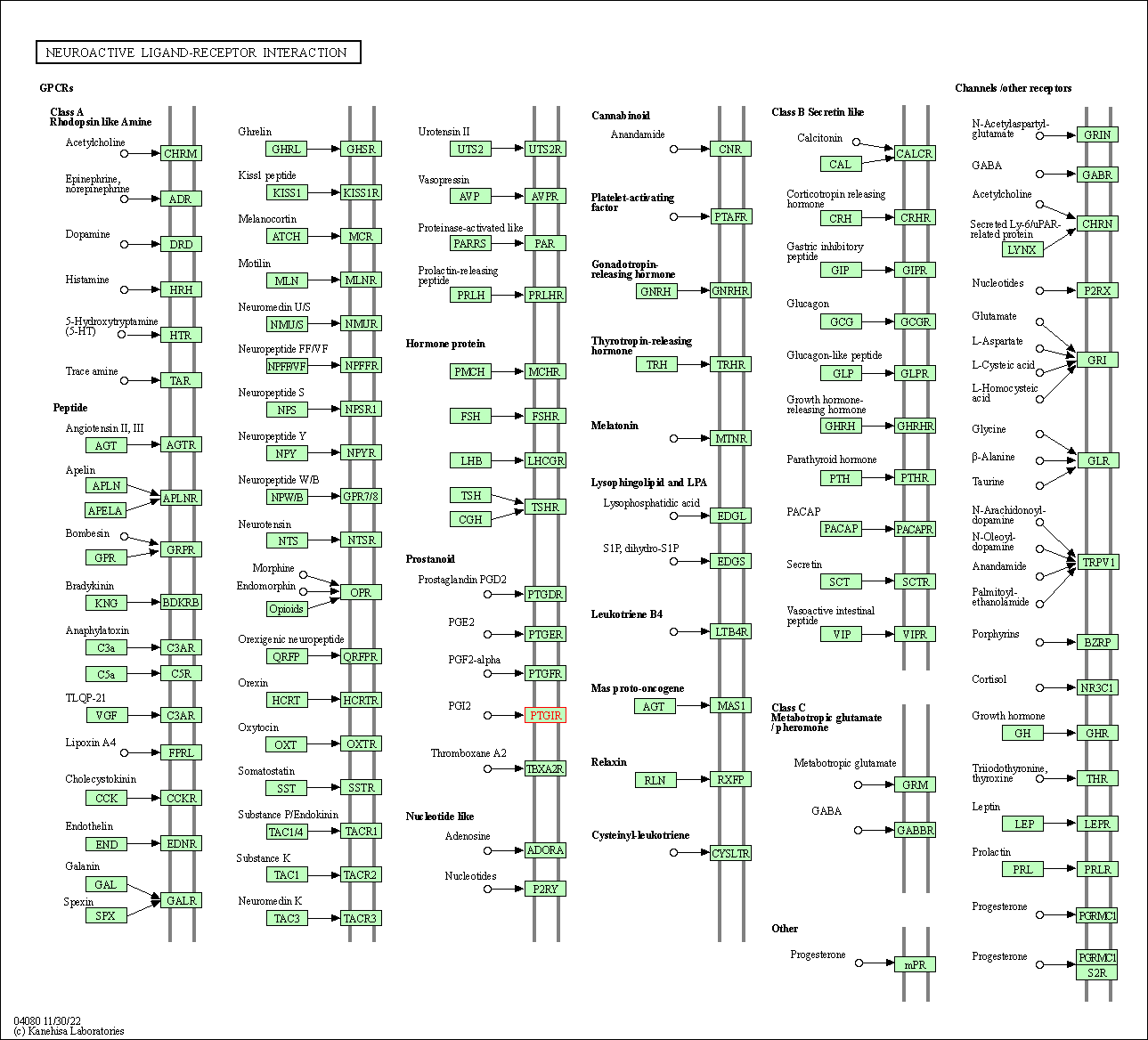
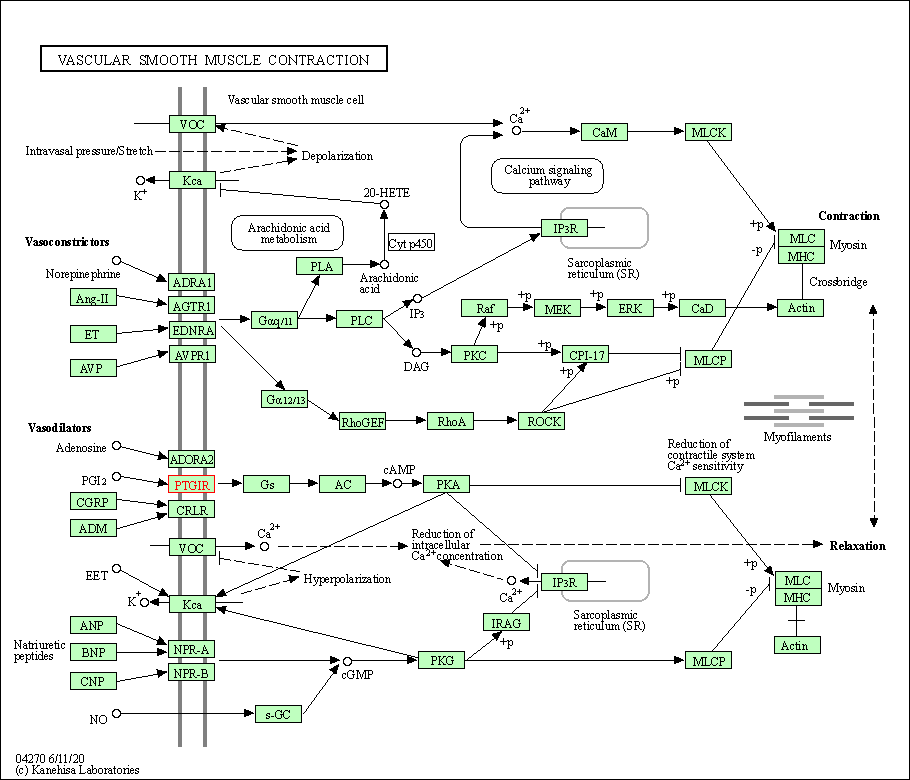
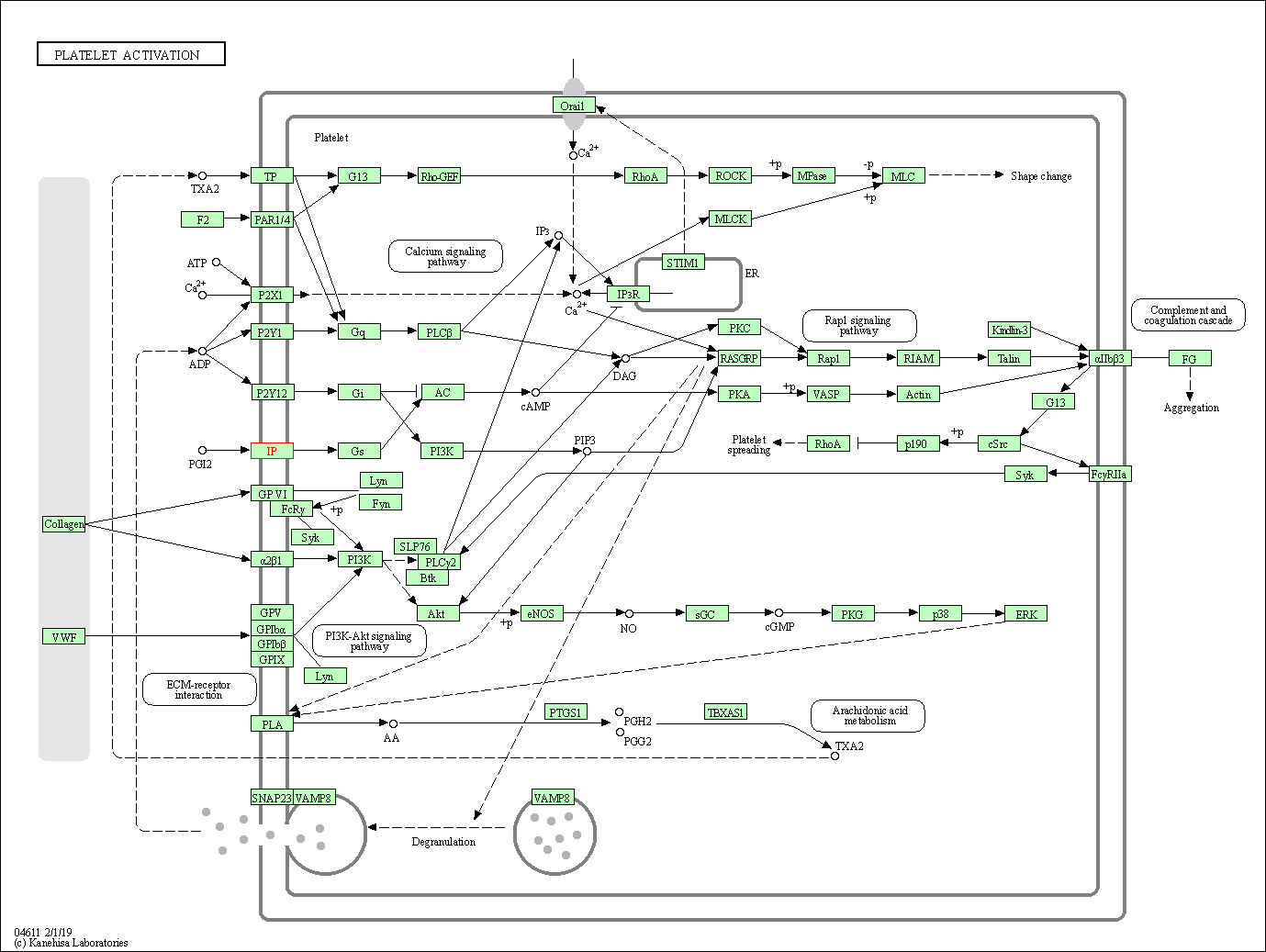
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Vascular smooth muscle contraction | hsa04270 | Affiliated Target |

|
| Class: Organismal Systems => Circulatory system | Pathway Hierarchy | ||
| Platelet activation | hsa04611 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 2 | Degree centrality | 2.15E-04 | Betweenness centrality | 2.88E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 1.83E-01 | Radiality | 1.31E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 2.55E+01 | Topological coefficient | 5.00E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 3 KEGG Pathways | + | ||||
| 1 | Neuroactive ligand-receptor interaction | |||||
| 2 | Vascular smooth muscle contraction | |||||
| 3 | Platelet activation | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Intracellular Signalling Through Prostacyclin Receptor and Prostacyclin | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | Thromboxane A2 receptor signaling | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Prostanoid ligand receptors | |||||
| 2 | G alpha (s) signalling events | |||||
| WikiPathways | [+] 7 WikiPathways | + | ||||
| 1 | Prostaglandin Synthesis and Regulation | |||||
| 2 | GPCRs, Class A Rhodopsin-like | |||||
| 3 | Small Ligand GPCRs | |||||
| 4 | Endothelin Pathways | |||||
| 5 | Platelet homeostasis | |||||
| 6 | GPCR ligand binding | |||||
| 7 | GPCR downstream signaling | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Palmitoylation of the human prostacyclin receptor. Functional implications of palmitoylation and isoprenylation. J Biol Chem. 2003 Feb 28;278(9):6947-58. | |||||
| REF 2 | Drug information of Dinoprost Tromethamine, 2008. eduDrugs. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1915). | |||||
| REF 4 | Emerging treatments for pulmonary arterial hypertension. Expert Opin Emerg Drugs. 2006 Nov;11(4):609-19. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1936). | |||||
| REF 6 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7552). | |||||
| REF 8 | ClinicalTrials.gov (NCT01106014) Selexipag (ACT-293987) in Pulmonary Arterial Hypertension, GRIPHON Trial. U.S. National Institutes of Health. | |||||
| REF 9 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5820). | |||||
| REF 10 | ClinicalTrials.gov (NCT03431649) Efficacy of Beraprost in Lowering Pulmonary Arterial Pressure in Children. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT01126073) A Double Blind, Randomized Study to Compare Influence of Niacin/Laropiprant on Functional and Morphological Characteristics of Arterial Wall and Parameters of Inflammation in Subjects With Coronary Heart Disease Already Treated With a Statin in Miran Sebestjen, University Medical Centre Ljubljana. | |||||
| REF 12 | Specific binding of the new stable epoprostenol analogue beraprost sodium to prostacyclin receptors on human and rat platelets. Arzneimittelforschung. 1989 Apr;39(4):495-9. | |||||
| REF 13 | ClinicalTrials.gov (NCT02279745) Long Term Safety and Efficacy of APD811 in Pulmonary Arterial Hypertension. U.S. National Institutes of Health. | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003132) | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002073) | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026064) | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005417) | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003249) | |||||
| REF 19 | Molecular cloning of human prostacyclin receptor cDNA and its gene expression in the cardiovascular system. Circulation. 1994 Oct;90(4):1643-7. | |||||
| REF 20 | Novel signaling pathways promote a paracrine wave of prostacyclin-induced vascular smooth muscle differentiation. J Mol Cell Cardiol. 2009 May;46(5):682-94. | |||||
| REF 21 | Signaling through Gi family members in platelets. Redundancy and specificity in the regulation of adenylyl cyclase and other effectors. J Biol Chem. 2002 Nov 29;277(48):46035-42. | |||||
| REF 22 | Expression of human hexosaminidase-A phenotype depends on genes assigned to chromosomes 5 and 15. Birth Defects Orig Artic Ser. 1976;12(7):192-6. | |||||
| REF 23 | The future potential of eicosanoids and their inhibitors in paediatric practice. Drugs. 1998 Aug;56(2):169-76. | |||||
| REF 24 | Structural organization and chromosomal assignment of the human prostacyclin receptor gene. Genomics. 1995 May 1;27(1):142-8. | |||||
| REF 25 | Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. 2012 Oct;40(4):874-80. | |||||
| REF 26 | Dosing considerations in the use of intravenous prostanoids in pulmonary arterial hypertension: an experience-based review. Am Heart J. 2009 Apr;157(4):625-35. | |||||
| REF 27 | Prostaglandin I2 IP Receptor Agonist, Beraprost, Prevents Transient Global Cerebral Ischemia Induced Hippocampal CA1 Injury in Aging Mice. J Neurol Disord. 2014;2:1000174. | |||||
| REF 28 | Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydroc... J Med Chem. 2007 Feb 22;50(4):794-806. | |||||
| REF 29 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 30 | Protective effect of a prostaglandin I2 analog, TEI-7165, on ischemic neuronal damage in gerbils. Brain Res. 1997 Sep 26;769(2):321-8. | |||||
| REF 31 | Pharmacokinetics of intravenous ataprost alfadex, a new prostaglandin I2 analog in healthy volunteers. Int J Clin Pharmacol Ther Toxicol. 1993 Aug;31(8):373-5. | |||||
| REF 32 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 345). | |||||
| REF 33 | Effect of a prostaglandin I(2) analog on the expression of thrombomodulin in liver and spleen endothelial cells after an extensive hepatectomy. Surg Today. 2011 Feb;41(2):230-6. | |||||
| REF 34 | Molecular design of novel PGI2 agonists without PG skeleton, Bioorg. Med. Chem. Lett. 5(10):1071-1076 (1995). | |||||
| REF 35 | Bioactivation of eptaloprost in animals and man. Prostaglandins. 1993 Aug;46(2):177-89. | |||||
| REF 36 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 37 | 7,7-Difluoroprostacyclin derivative, AFP-07, a highly selective and potent agonist for the prostacyclin receptor. Prostaglandins. 1997 Feb;53(2):83-90. | |||||
| REF 38 | Vascular actions of the prostacyclin receptor antagonist BAY 73-1449 in the portal hypertensive rat. Eur J Pharmacol. 2008 Aug 20;590(1-3):322-6. | |||||
| REF 39 | Relaxant actions of nonprostanoid prostacyclin mimetics on human pulmonary artery. J Cardiovasc Pharmacol. 1997 Apr;29(4):525-35. | |||||
| REF 40 | The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000 Jan 17;1483(2):285-93. | |||||
| REF 41 | Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997 Sep;122(2):217-24. | |||||
| REF 42 | Prostaglandin endoperoxide analogues which are both thromboxane receptor antagonists and prostacyclin mimetics. Br J Pharmacol. 1986 Mar;87(3):543-51. | |||||
| REF 43 | Replacing the cyclohexene-linker of FR181157 leading to novel IP receptor agonists: orally active prostacyclin mimetics. Part 6. Bioorg Med Chem Lett. 2006 Sep 15;16(18):4861-4. | |||||
| REF 44 | Discovery of new diphenyloxazole derivatives containing a pyrrolidine ring: orally active prostacyclin mimetics. Part 2. Bioorg Med Chem Lett. 2005 Jul 1;15(13):3279-83. | |||||
| REF 45 | RO1138452 and RO3244794: characterization of structurally distinct, potent and selective IP (prostacyclin) receptor antagonists. Br J Pharmacol. 2006 Feb;147(3):335-45. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

