Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0M4TJ
|
|||
| Drug Name |
Ralinepag
|
|||
| Synonyms |
UNII-CQY12ZJN6E; CQY12ZJN6E; 1187856-49-0; Ralinepag [USAN:INN]; Ralinepag (USAN/INN); SCHEMBL1118504; SCHEMBL1118506; SCHEMBL12786473; CHEMBL3919269; CHEMBL3301604; AKOS027337124; DB12462; 2-((trans-4-((((4-Chlorophenyl)(phenyl)carbamoyl)oxy)methyl)cyclohexyl)methoxy)acetic acid; Acetic acid, 2-((trans-4-(((((4-chlorophenyl)phenylamino)carbonyl)oxy)methyl)cyclohexyl)methoxy)-; HY-16751; CS-0012350; J3.614.088G; D10725
Click to Show/Hide
|
|||
| Indication | Pulmonary arterial hypertension [ICD-11: BB01.0; ICD-9: 416] | Phase 2 | [1], [2] | |
| Pulmonary hypertension [ICD-11: BB01; ICD-10: I27, I27.0; ICD-9: 416] | Phase 2 | [3] | ||
| Company |
Arena Pharmaceuticals San Diego, CA
|
|||
| Structure |
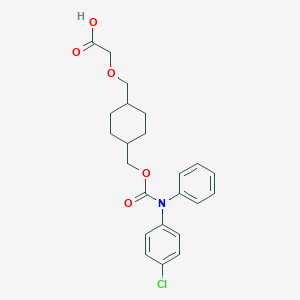 |
Download2D MOL |
||
| Formula |
C23H26ClNO5
|
|||
| Canonical SMILES |
C1CC(CCC1COCC(=O)O)COC(=O)N(C2=CC=CC=C2)C3=CC=C(C=C3)Cl
|
|||
| InChI |
1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)
|
|||
| InChIKey |
NPDKXVKJRHPDQT-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1187856-49-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Prostacyclin receptor (PTGIR) | Target Info | Agonist | [1] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Vascular smooth muscle contraction | ||||
| Platelet activation | ||||
| Pathwhiz Pathway | Intracellular Signalling Through Prostacyclin Receptor and Prostacyclin | |||
| Pathway Interaction Database | Thromboxane A2 receptor signaling | |||
| Reactome | Prostanoid ligand receptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Prostaglandin Synthesis and Regulation | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Small Ligand GPCRs | ||||
| Endothelin Pathways | ||||
| Platelet homeostasis | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | ClinicalTrials.gov (NCT02279745) Long Term Safety and Efficacy of APD811 in Pulmonary Arterial Hypertension. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

