Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T07173
(Former ID: TTDC00318)
|
|||||
| Target Name |
Tropomyosin-related kinase A (TrkA)
|
|||||
| Synonyms |
gp140trk; Tyrosine kinase receptor A; Tyrosine kinase receptor; Trk-A; TRKA; TRK1-transforming tyrosine kinase protein; TRK1 transforming tyrosinekinase protein; TRK; P140-TrkA; Neurotrophic tyrosine kinase receptor type 1; NGF-trk receptor type A; MTC; High affinity nerve growth factor receptor
Click to Show/Hide
|
|||||
| Gene Name |
NTRK1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Non-small-cell lung cancer [ICD-11: 2C25] | |||||
| 2 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
High affinity receptor for NGF which is its primary ligand. Can also bind and be activated by NTF3/neurotrophin-3. However, NTF3 only supports axonal extension through NTRK1 but has no effect on neuron survival. Upon dimeric NGF ligand-binding, undergoes homodimerization, autophosphorylation and activation. Recruits, phosphorylates and/or activates several downstream effectors including SHC1, FRS2, SH2B1, SH2B2 and PLCG1 that regulate distinct overlapping signaling cascades driving cell survival and differentiation. Through SHC1 and FRS2 activates a GRB2-Ras-MAPK cascade that regulates cell differentiation and survival. Through PLCG1 controls NF-Kappa-B activation and the transcription of genes involved in cell survival. Through SHC1 and SH2B1 controls a Ras-PI3 kinase-AKT1 signaling cascade that is also regulating survival. In absence of ligand and activation, may promote cell death, making the survival of neurons dependent on trophic factors. Receptor tyrosine kinase involved in the development and the maturation of the central and peripheral nervous systems through regulation of proliferation, differentiation and survival of sympathetic and nervous neurons.
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.10.1
|
|||||
| Sequence |
MLRGGRRGQLGWHSWAAGPGSLLAWLILASAGAAPCPDACCPHGSSGLRCTRDGALDSLH
HLPGAENLTELYIENQQHLQHLELRDLRGLGELRNLTIVKSGLRFVAPDAFHFTPRLSRL NLSFNALESLSWKTVQGLSLQELVLSGNPLHCSCALRWLQRWEEEGLGGVPEQKLQCHGQ GPLAHMPNASCGVPTLKVQVPNASVDVGDDVLLRCQVEGRGLEQAGWILTELEQSATVMK SGGLPSLGLTLANVTSDLNRKNVTCWAENDVGRAEVSVQVNVSFPASVQLHTAVEMHHWC IPFSVDGQPAPSLRWLFNGSVLNETSFIFTEFLEPAANETVRHGCLRLNQPTHVNNGNYT LLAANPFGQASASIMAAFMDNPFEFNPEDPIPVSFSPVDTNSTSGDPVEKKDETPFGVSV AVGLAVFACLFLSTLLLVLNKCGRRNKFGINRPAVLAPEDGLAMSLHFMTLGGSSLSPTE GKGSGLQGHIIENPQYFSDACVHHIKRRDIVLKWELGEGAFGKVFLAECHNLLPEQDKML VAVKALKEASESARQDFQREAELLTMLQHQHIVRFFGVCTEGRPLLMVFEYMRHGDLNRF LRSHGPDAKLLAGGEDVAPGPLGLGQLLAVASQVAAGMVYLAGLHFVHRDLATRNCLVGQ GLVVKIGDFGMSRDIYSTDYYRVGGRTMLPIRWMPPESILYRKFTTESDVWSFGVVLWEI FTYGKQPWYQLSNTEAIDCITQGRELERPRACPPEVYAIMRGCWQREPQQRHSIKDVHAR LQALAQAPPVYLDVLG Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T75OVL | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 3 Approved Drugs | + | ||||
| 1 | Entrectinib | Drug Info | Approved | Non-small-cell lung cancer | [2] | |
| 2 | Larotrectinib | Drug Info | Approved | Solid tumour/cancer | [3] | |
| 3 | Repotrectinib | Drug Info | Approved | Non-small-cell lung cancer | [4] | |
| Clinical Trial Drug(s) | [+] 9 Clinical Trial Drugs | + | ||||
| 1 | MIM-D3 | Drug Info | Phase 3 | Alzheimer disease | [5] | |
| 2 | CT 327 | Drug Info | Phase 2 | Psoriasis vulgaris | [6] | |
| 3 | SNA-120 | Drug Info | Phase 2 | Plaque psoriasis | [7] | |
| 4 | ONO-7579 | Drug Info | Phase 1/2 | Solid tumour/cancer | [3] | |
| 5 | Altiratinib | Drug Info | Phase 1 | Solid tumour/cancer | [8] | |
| 6 | DS-6051 | Drug Info | Phase 1 | Solid tumour/cancer | [9] | |
| 7 | LOXO-195 | Drug Info | Phase 1 | Solid tumour/cancer | [3] | |
| 8 | PLX7486 | Drug Info | Phase 1 | Pancreatic cancer | [10] | |
| 9 | VMD-928 | Drug Info | Phase 1 | Lymphoma | [11] | |
| Patented Agent(s) | [+] 74 Patented Agents | + | ||||
| 1 | 3-amino-5-benzyl-substituted indazole derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 2 | Azaindazole amide derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 3 | Benzimidazole derivative 6 | Drug Info | Patented | Solid tumour/cancer | [13] | |
| 4 | Bicyclic heteroaryl benzamide derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 5 | Bicyclic heteroaryl benzamide derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| 6 | Bicyclic heteroaryl benzamide derivative 3 | Drug Info | Patented | Pruritus | [12] | |
| 7 | Bicyclic heteroaryl benzamide derivative 4 | Drug Info | Patented | Pruritus | [12] | |
| 8 | Bicyclic heteroaryl benzamide derivative 5 | Drug Info | Patented | Pruritus | [12] | |
| 9 | Bicyclic heteroaryl benzamide derivative 6 | Drug Info | Patented | Pruritus | [12] | |
| 10 | Bicyclic heteroaryl benzamide derivative 7 | Drug Info | Patented | Pruritus | [12] | |
| 11 | Bicyclic heteroaryl benzamide derivative 8 | Drug Info | Patented | Pruritus | [12] | |
| 12 | Bicyclic heteroaryl benzamide derivative 9 | Drug Info | Patented | Pruritus | [12] | |
| 13 | Five membered heterocyclic benzamide derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 14 | Five membered heterocyclic benzamide derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| 15 | Five membered heterocyclic benzamide derivative 3 | Drug Info | Patented | Pruritus | [12] | |
| 16 | Imidazo pyridine derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 17 | Imidazo[1,2-b]pyridazine derivative 1 | Drug Info | Patented | Solid tumour/cancer | [13] | |
| 18 | Imidazo[1,2-b]pyridazine derivative 2 | Drug Info | Patented | Solid tumour/cancer | [13] | |
| 19 | Imidazo[1,2-b]pyridazine derivative 3 | Drug Info | Patented | Solid tumour/cancer | [13] | |
| 20 | N-(phenylpyrazolyl)benzamide derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 21 | N-acylpiperidine ether derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 22 | N-acylpiperidine ether derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| 23 | N-acylpiperidine ether derivative 3 | Drug Info | Patented | Pruritus | [12] | |
| 24 | N-acylpiperidine ether derivative 4 | Drug Info | Patented | Pruritus | [12] | |
| 25 | N-acylpiperidine ether derivative 5 | Drug Info | Patented | Pruritus | [12] | |
| 26 | N-acylpiperidine ether derivative 6 | Drug Info | Patented | Pruritus | [12] | |
| 27 | N-acylpiperidine ether derivative 7 | Drug Info | Patented | Pruritus | [12] | |
| 28 | N-acylpyrrolidine ether derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 29 | N-acylpyrrolidine ether derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| 30 | PMID28270021-Compound-WO2010077680 103 | Drug Info | Patented | Pruritus | [12] | |
| 31 | PMID28270021-Compound-WO2010077680 109 | Drug Info | Patented | Pruritus | [12] | |
| 32 | PMID28270021-Compound-WO2010077680 201 | Drug Info | Patented | Pruritus | [12] | |
| 33 | PMID28270021-Compound-WO2010077680 481 | Drug Info | Patented | Pruritus | [12] | |
| 34 | PMID28270021-Compound-WO2010077680 495 | Drug Info | Patented | Pruritus | [12] | |
| 35 | PMID28270021-Compound-WO2010077680 803 | Drug Info | Patented | Pruritus | [12] | |
| 36 | PMID28270021-Compound-WO2010077680 811 | Drug Info | Patented | Pruritus | [12] | |
| 37 | PMID28270021-Compound-WO2013009582Example16 | Drug Info | Patented | Pruritus | [12] | |
| 38 | PMID28270021-Compound-WO2013009582Example76 | Drug Info | Patented | Pruritus | [12] | |
| 39 | PMID28270021-Compound-WO2013161919Example85-117 | Drug Info | Patented | Pruritus | [12] | |
| 40 | PMID28270021-Compound-WO2014078408Example1 | Drug Info | Patented | Pruritus | [12] | |
| 41 | PMID28270021-Compound-WO2014078408Example26 | Drug Info | Patented | Pruritus | [12] | |
| 42 | PMID28270021-Compound-WO2014129431Example8-1 | Drug Info | Patented | Pruritus | [12] | |
| 43 | PMID28270021-Compound-WO2014152663 701 | Drug Info | Patented | Osteoarthritis pain | [12] | |
| 44 | PMID28270021-Compound-WO2015042088Example11 | Drug Info | Patented | Pruritus | [12] | |
| 45 | PMID28270021-Compound-WO2015042088Example12 | Drug Info | Patented | Pruritus | [12] | |
| 46 | PMID28270021-Compound-WO2015042088Example4 | Drug Info | Patented | Pruritus | [12] | |
| 47 | PMID28270021-Compound-WO2016054807Example1 | Drug Info | Patented | Pruritus | [12] | |
| 48 | PMID28270021-Compound-WO2016054807Example112 | Drug Info | Patented | Pruritus | [12] | |
| 49 | PMID28270021-Compound-WO2016054807Example71 | Drug Info | Patented | Pruritus | [12] | |
| 50 | PMID28270021-Compound-WO2016054807Example80 | Drug Info | Patented | Pruritus | [12] | |
| 51 | PMID28270021-Compound-WO2016054807Example82 | Drug Info | Patented | Pruritus | [12] | |
| 52 | Pyrazolo[1,5-a]pyridine derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 53 | Pyrazolo[1,5-a]pyridine derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| 54 | Pyrazolo[4,3-c]pyridine derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 55 | Pyrazolo[4,3-h]quinazoline-3-carboxamide derivative 1 | Drug Info | Patented | Malignant thymoma | [12] | |
| 56 | Pyrido[3,2-d]pyrimidine derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| 57 | Pyrido[3,2-d]pyrimidine derivative 3 | Drug Info | Patented | Pruritus | [12] | |
| 58 | Pyrrolo[2,3-b]pyridine derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 59 | Pyrrolo[2,3-b]pyridine derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| 60 | Pyrrolo[2,3-b]pyridine derivative 3 | Drug Info | Patented | Pruritus | [12] | |
| 61 | Pyrrolo[2,3-d]pyrimidine derivative 3 | Drug Info | Patented | Pruritus | [12] | |
| 62 | Pyrrolo[2,3-d]pyrimidine derivative 4 | Drug Info | Patented | Pruritus | [12] | |
| 63 | Pyrrolo[3,2-c]pyridine derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 64 | Six-membered heterocyclic benzamide derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 65 | Six-membered heterocyclic benzamide derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| 66 | Six-membered heterocyclic benzamide derivative 3 | Drug Info | Patented | Pruritus | [12] | |
| 67 | Six-membered heterocyclic benzamide derivative 4 | Drug Info | Patented | Pruritus | [12] | |
| 68 | Six-membered heterocyclic benzamide derivative 5 | Drug Info | Patented | Pruritus | [12] | |
| 69 | Six-membered heterocyclic benzamide derivative 6 | Drug Info | Patented | Pruritus | [12] | |
| 70 | Six-membered heterocyclic benzamide derivative 7 | Drug Info | Patented | Pruritus | [12] | |
| 71 | Thiadiazolyl carboxamide derivative 1 | Drug Info | Patented | Alzheimer disease | [13] | |
| 72 | Tri-substituted urea derivative 1 | Drug Info | Patented | Pruritus | [12] | |
| 73 | Tri-substituted urea derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| 74 | Triazolo[4,3-b]pyridazine derivative 2 | Drug Info | Patented | Pruritus | [12] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | AZD6918 | Drug Info | Discontinued in Phase 1 | Advanced solid tumour | [14] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | FX-007 | Drug Info | Preclinical | Pain | [15] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Inhibitor | [+] 131 Inhibitor drugs | + | ||||
| 1 | Entrectinib | Drug Info | [2] | |||
| 2 | Larotrectinib | Drug Info | [1], [16] | |||
| 3 | Repotrectinib | Drug Info | [4] | |||
| 4 | CT 327 | Drug Info | [18] | |||
| 5 | SNA-120 | Drug Info | [7] | |||
| 6 | ONO-7579 | Drug Info | [3] | |||
| 7 | Altiratinib | Drug Info | [13] | |||
| 8 | DS-6051 | Drug Info | [19] | |||
| 9 | LOXO-195 | Drug Info | [3] | |||
| 10 | PLX7486 | Drug Info | [3], [20] | |||
| 11 | VMD-928 | Drug Info | [11] | |||
| 12 | 3-amino-5-benzyl-substituted indazole derivative 1 | Drug Info | [12] | |||
| 13 | Azaindazole amide derivative 1 | Drug Info | [12] | |||
| 14 | Benzimidazole derivative 6 | Drug Info | [13] | |||
| 15 | Bicyclic heteroaryl benzamide derivative 1 | Drug Info | [12] | |||
| 16 | Bicyclic heteroaryl benzamide derivative 2 | Drug Info | [12] | |||
| 17 | Bicyclic heteroaryl benzamide derivative 3 | Drug Info | [12] | |||
| 18 | Bicyclic heteroaryl benzamide derivative 4 | Drug Info | [12] | |||
| 19 | Bicyclic heteroaryl benzamide derivative 5 | Drug Info | [12] | |||
| 20 | Bicyclic heteroaryl benzamide derivative 6 | Drug Info | [12] | |||
| 21 | Bicyclic heteroaryl benzamide derivative 7 | Drug Info | [12] | |||
| 22 | Bicyclic heteroaryl benzamide derivative 8 | Drug Info | [12] | |||
| 23 | Bicyclic heteroaryl benzamide derivative 9 | Drug Info | [12] | |||
| 24 | Five membered heterocyclic benzamide derivative 1 | Drug Info | [12] | |||
| 25 | Five membered heterocyclic benzamide derivative 2 | Drug Info | [12] | |||
| 26 | Five membered heterocyclic benzamide derivative 3 | Drug Info | [12] | |||
| 27 | Imidazo pyridine derivative 1 | Drug Info | [12] | |||
| 28 | Imidazo[1,2-b]pyridazine derivative 1 | Drug Info | [13] | |||
| 29 | Imidazo[1,2-b]pyridazine derivative 2 | Drug Info | [13] | |||
| 30 | Imidazo[1,2-b]pyridazine derivative 3 | Drug Info | [13] | |||
| 31 | Imidazo[1,2-b]pyridazine derivative 4 | Drug Info | [13] | |||
| 32 | Imidazo[1,2-b]pyridazine derivative 5 | Drug Info | [13] | |||
| 33 | Imidazo[1,2-b]pyridazine derivative 6 | Drug Info | [13] | |||
| 34 | Imidazo[1,2-b]pyridazine derivative 7 | Drug Info | [13] | |||
| 35 | N,N-bis(5-pyrazoyl)urea derivative 1 | Drug Info | [13] | |||
| 36 | N-(phenylpyrazolyl)benzamide derivative 1 | Drug Info | [12] | |||
| 37 | N-acylpiperidine ether derivative 1 | Drug Info | [12] | |||
| 38 | N-acylpiperidine ether derivative 2 | Drug Info | [12] | |||
| 39 | N-acylpiperidine ether derivative 3 | Drug Info | [12] | |||
| 40 | N-acylpiperidine ether derivative 4 | Drug Info | [12] | |||
| 41 | N-acylpiperidine ether derivative 5 | Drug Info | [12] | |||
| 42 | N-acylpiperidine ether derivative 6 | Drug Info | [12] | |||
| 43 | N-acylpiperidine ether derivative 7 | Drug Info | [12] | |||
| 44 | N-acylpyrrolidine ether derivative 1 | Drug Info | [12] | |||
| 45 | N-acylpyrrolidine ether derivative 2 | Drug Info | [12] | |||
| 46 | N-arylmethyl-N-phenyl cyclic urea derivative 1 | Drug Info | [13] | |||
| 47 | N-arylmethyl-N-phenyl cyclic urea derivative 2 | Drug Info | [13] | |||
| 48 | PMID28270010-Compound-Figure16-a | Drug Info | [13] | |||
| 49 | PMID28270010-Compound-Figure16-b-1 | Drug Info | [13] | |||
| 50 | PMID28270010-Compound-Figure16-b-2 | Drug Info | [13] | |||
| 51 | PMID28270010-Compound-Figure17-1 | Drug Info | [13] | |||
| 52 | PMID28270010-Compound-Figure17-2 | Drug Info | [13] | |||
| 53 | PMID28270010-Compound-Figure17-3 | Drug Info | [13] | |||
| 54 | PMID28270010-Compound-Figure24-a | Drug Info | [13] | |||
| 55 | PMID28270010-Compound-Figure24-b | Drug Info | [13] | |||
| 56 | PMID28270010-Compound-Figure5-1 | Drug Info | [13] | |||
| 57 | PMID28270010-Compound-Figure5-2 | Drug Info | [13] | |||
| 58 | PMID28270010-Compound-Figure5-3 | Drug Info | [13] | |||
| 59 | PMID28270021-Compound-WO2013009582Example16 | Drug Info | [12] | |||
| 60 | PMID28270021-Compound-WO2013009582Example76 | Drug Info | [12] | |||
| 61 | PMID28270021-Compound-WO2013161919Example85-117 | Drug Info | [12] | |||
| 62 | PMID28270021-Compound-WO2014078408Example1 | Drug Info | [12] | |||
| 63 | PMID28270021-Compound-WO2014078408Example26 | Drug Info | [12] | |||
| 64 | PMID28270021-Compound-WO2014129431Example8-1 | Drug Info | [12] | |||
| 65 | PMID28270021-Compound-WO2014152663 701 | Drug Info | [12] | |||
| 66 | PMID28270021-Compound-WO2015042088Example11 | Drug Info | [12] | |||
| 67 | PMID28270021-Compound-WO2015042088Example12 | Drug Info | [12] | |||
| 68 | PMID28270021-Compound-WO2015042088Example4 | Drug Info | [12] | |||
| 69 | PMID28270021-Compound-WO2016054807Example1 | Drug Info | [12] | |||
| 70 | PMID28270021-Compound-WO2016054807Example112 | Drug Info | [12] | |||
| 71 | PMID28270021-Compound-WO2016054807Example71 | Drug Info | [12] | |||
| 72 | PMID28270021-Compound-WO2016054807Example80 | Drug Info | [12] | |||
| 73 | PMID28270021-Compound-WO2016054807Example82 | Drug Info | [12] | |||
| 74 | Pyrazolo[1,5-a]pyridine derivative 1 | Drug Info | [12] | |||
| 75 | Pyrazolo[1,5-a]pyridine derivative 2 | Drug Info | [12] | |||
| 76 | Pyrazolo[1,5-a]pyrimidine derivative 14 | Drug Info | [13] | |||
| 77 | Pyrazolo[1,5-a]pyrimidine derivative 15 | Drug Info | [13] | |||
| 78 | Pyrazolo[1,5-a]pyrimidine derivative 16 | Drug Info | [13] | |||
| 79 | Pyrazolo[1,5-a]pyrimidine derivative 17 | Drug Info | [13] | |||
| 80 | Pyrazolo[1,5-a]pyrimidine derivative 18 | Drug Info | [13] | |||
| 81 | Pyrazolo[1,5-a]pyrimidine derivative 19 | Drug Info | [13] | |||
| 82 | Pyrazolo[1,5-a]pyrimidine derivative 20 | Drug Info | [13] | |||
| 83 | Pyrazolo[1,5-a]pyrimidine derivative 21 | Drug Info | [13] | |||
| 84 | Pyrazolo[1,5-a]pyrimidine derivative 22 | Drug Info | [13] | |||
| 85 | Pyrazolo[1,5-a]pyrimidine derivative 23 | Drug Info | [13] | |||
| 86 | Pyrazolo[1,5-a]pyrimidine derivative 24 | Drug Info | [13] | |||
| 87 | Pyrazolo[1,5-a]pyrimidine derivative 25 | Drug Info | [13] | |||
| 88 | Pyrazolo[1,5-a]pyrimidine derivative 26 | Drug Info | [13] | |||
| 89 | Pyrazolo[1,5-a]pyrimidine derivative 27 | Drug Info | [13] | |||
| 90 | Pyrazolo[4,3-c]pyridine derivative 1 | Drug Info | [12] | |||
| 91 | Pyrazolo[4,3-h]quinazoline-3-carboxamide derivative 1 | Drug Info | [12] | |||
| 92 | Pyrido[3,2-d]pyrimidine derivative 2 | Drug Info | [12] | |||
| 93 | Pyrido[3,2-d]pyrimidine derivative 3 | Drug Info | [12] | |||
| 94 | Pyrrolidinyl urea derivative 10 | Drug Info | [13] | |||
| 95 | Pyrrolidinyl urea derivative 11 | Drug Info | [13] | |||
| 96 | Pyrrolidinyl urea derivative 12 | Drug Info | [13] | |||
| 97 | Pyrrolidinyl urea derivative 13 | Drug Info | [13] | |||
| 98 | Pyrrolidinyl urea derivative 2 | Drug Info | [13] | |||
| 99 | Pyrrolidinyl urea derivative 3 | Drug Info | [13] | |||
| 100 | Pyrrolidinyl urea derivative 4 | Drug Info | [13] | |||
| 101 | Pyrrolidinyl urea derivative 5 | Drug Info | [13] | |||
| 102 | Pyrrolidinyl urea derivative 6 | Drug Info | [13] | |||
| 103 | Pyrrolidinyl urea derivative 7 | Drug Info | [13] | |||
| 104 | Pyrrolidinyl urea derivative 8 | Drug Info | [13] | |||
| 105 | Pyrrolidinyl urea derivative 9 | Drug Info | [13] | |||
| 106 | Pyrrolo[2,3-b]pyridine derivative 1 | Drug Info | [12] | |||
| 107 | Pyrrolo[2,3-b]pyridine derivative 2 | Drug Info | [12] | |||
| 108 | Pyrrolo[2,3-b]pyridine derivative 3 | Drug Info | [12] | |||
| 109 | Pyrrolo[2,3-d]pyrimidine derivative 3 | Drug Info | [12] | |||
| 110 | Pyrrolo[2,3-d]pyrimidine derivative 4 | Drug Info | [12] | |||
| 111 | Pyrrolo[3,2-c]pyridine derivative 1 | Drug Info | [12] | |||
| 112 | Six-membered heterocyclic benzamide derivative 1 | Drug Info | [12] | |||
| 113 | Six-membered heterocyclic benzamide derivative 2 | Drug Info | [12] | |||
| 114 | Six-membered heterocyclic benzamide derivative 3 | Drug Info | [12] | |||
| 115 | Six-membered heterocyclic benzamide derivative 4 | Drug Info | [12] | |||
| 116 | Six-membered heterocyclic benzamide derivative 5 | Drug Info | [12] | |||
| 117 | Six-membered heterocyclic benzamide derivative 6 | Drug Info | [12] | |||
| 118 | Six-membered heterocyclic benzamide derivative 7 | Drug Info | [12] | |||
| 119 | Thiadiazolyl carboxamide derivative 1 | Drug Info | [13] | |||
| 120 | Thiazole derivative 4 | Drug Info | [13] | |||
| 121 | Tri-substituted urea derivative 1 | Drug Info | [12] | |||
| 122 | Tri-substituted urea derivative 2 | Drug Info | [12] | |||
| 123 | Triazolo[4,3-b]pyridazine derivative 2 | Drug Info | [12] | |||
| 124 | Ureido-phenyl-substituted triazine derivative 1 | Drug Info | [13] | |||
| 125 | Ureido-phenyl-substituted triazine derivative 2 | Drug Info | [13] | |||
| 126 | AZD6918 | Drug Info | [21] | |||
| 127 | FX-007 | Drug Info | [22] | |||
| 128 | CEP-5104 | Drug Info | [23] | |||
| 129 | CEP-6331 | Drug Info | [23] | |||
| 130 | GW-5074 | Drug Info | [24] | |||
| 131 | K-252a analogue | Drug Info | [25] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | MIM-D3 | Drug Info | [17] | |||
| Antagonist | [+] 7 Antagonist drugs | + | ||||
| 1 | PMID28270021-Compound-WO2010077680 103 | Drug Info | [12] | |||
| 2 | PMID28270021-Compound-WO2010077680 109 | Drug Info | [12] | |||
| 3 | PMID28270021-Compound-WO2010077680 201 | Drug Info | [12] | |||
| 4 | PMID28270021-Compound-WO2010077680 481 | Drug Info | [12] | |||
| 5 | PMID28270021-Compound-WO2010077680 495 | Drug Info | [12] | |||
| 6 | PMID28270021-Compound-WO2010077680 803 | Drug Info | [12] | |||
| 7 | PMID28270021-Compound-WO2010077680 811 | Drug Info | [12] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Entrectinib | Ligand Info | |||||
| Structure Description | THE STRUCTURE OF TRKA KINASE DOMAIN BOUND TO THE INHIBITOR ENTRECTINIB | PDB:5KVT | ||||
| Method | X-ray diffraction | Resolution | 2.45 Å | Mutation | No | [26] |
| PDB Sequence |
CVHHIKRRDI
510 VLKWELGEGA520 FGKVFLAECH530 NLDKMLVAVK544 ALKEASESAR554 QDFQREAELL 564 TMLQHQHIVR574 FFGVCTEGRP584 LLMVFEYMRH594 GDLNRFLRSH604 GPDAKLLAGG 614 EDVAPGPLGL624 GQLLAVASQV634 AAGMVYLAGL644 HFVHRDLATR654 NCLVGQGLVV 664 KIGDFGMSRD674 IYSTDYYRVG684 GRTMLPIRWM694 PPESILYRKF704 TTESDVWSFG 714 VVLWEIFTYG724 KQPWYQLSNT734 EAIDCITQGR744 ELERPRACPP754 EVYAIMRGCW 764 QREPQQRHSI774 KDVHARLQAL784 AQAHHHH
|
|||||
|
|
LEU516
3.361
GLY517
3.777
GLU518
3.286
GLY519
4.922
PHE521
3.916
VAL524
3.184
ALA542
3.335
LYS544
4.576
VAL573
4.062
PHE589
3.591
GLU590
2.872
TYR591
3.886
|
|||||
| Ligand Name: TPX-0005 | Ligand Info | |||||
| Structure Description | Crystal structure of TrkA (G595R) kinase with repotrectinib | PDB:7VKN | ||||
| Method | X-ray diffraction | Resolution | 2.70 Å | Mutation | Yes | [27] |
| PDB Sequence |
GSGIRVHHIK
506 RRDIVLKWEL516 GEGAFGKVFL526 AECHNLLPEQ536 DKMLVAVKAL546 KEASESARQD 556 FQREAELLTM566 LQHQHIVRFF576 GVCTEGRPLL586 MVFEYMRHRD596 LNRFLRSHGP 606 DAKLLAGGED616 VAPGPLGLGQ626 LLAVASQVAA636 GMVYLAGLHF646 VHRDLATRNC 656 LVGQGLVVKI666 GDFGMRDIYS677 TDYYRVGGRT687 MLPIRWMPPE697 SILYRKFTTE 707 SDVWSFGVVL717 WEIFTYGKQP727 WYQLSNTEAI737 DCITQGRELE747 RPRACPPEVY 757 AIMRGCWQRE767 PQQRHSIKDV777 HARLQALAQA787 PPVYLDV
|
|||||
|
|
LEU516
4.035
GLY517
3.681
GLU518
4.372
VAL524
3.795
ALA542
3.087
LYS544
4.277
VAL573
4.178
PHE589
3.930
GLU590
3.183
TYR591
3.820
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|

| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Ras signaling pathway | hsa04014 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Apoptosis | hsa04210 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
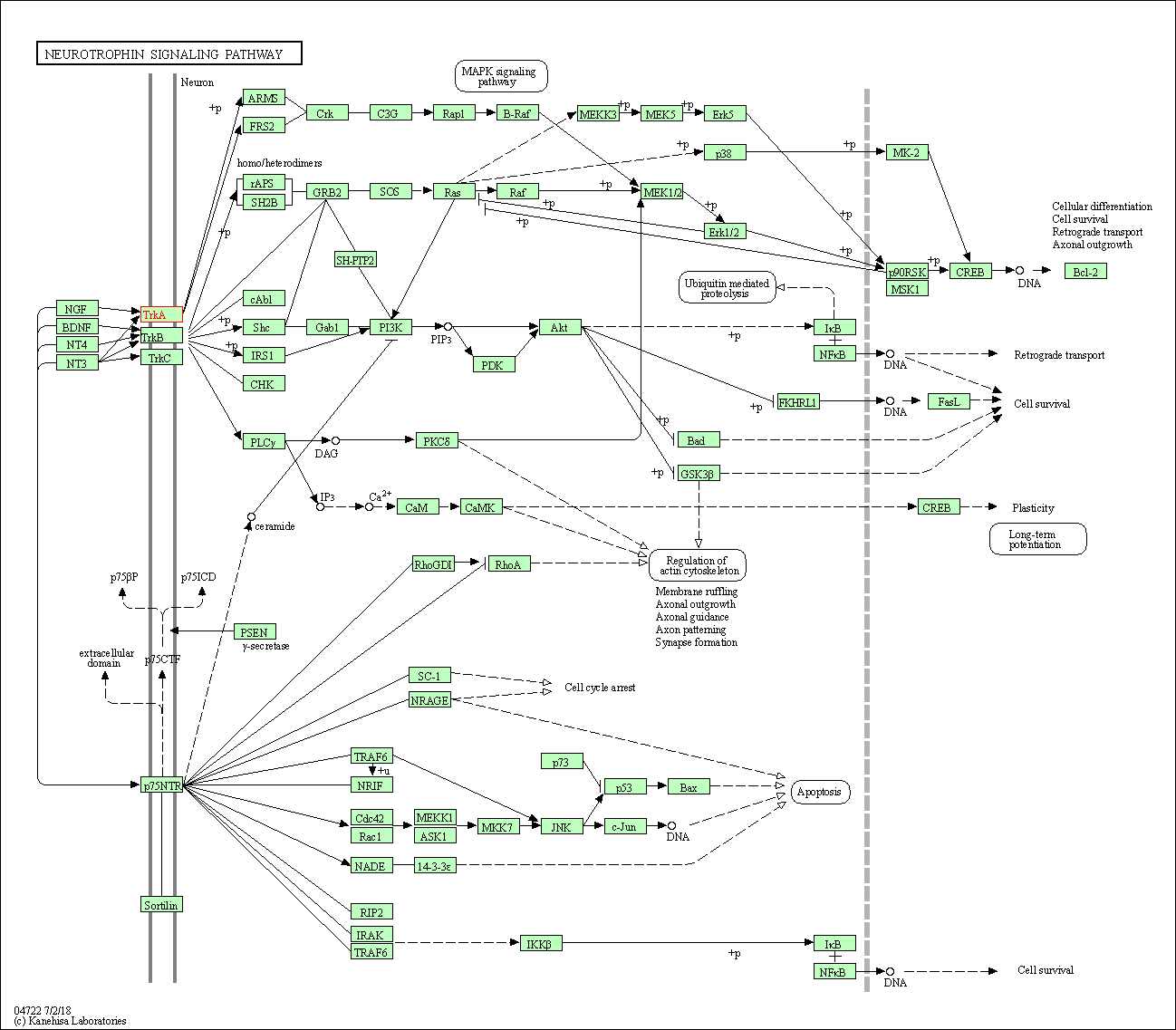
| Neurotrophin signaling pathway | hsa04722 | Affiliated Target |

|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
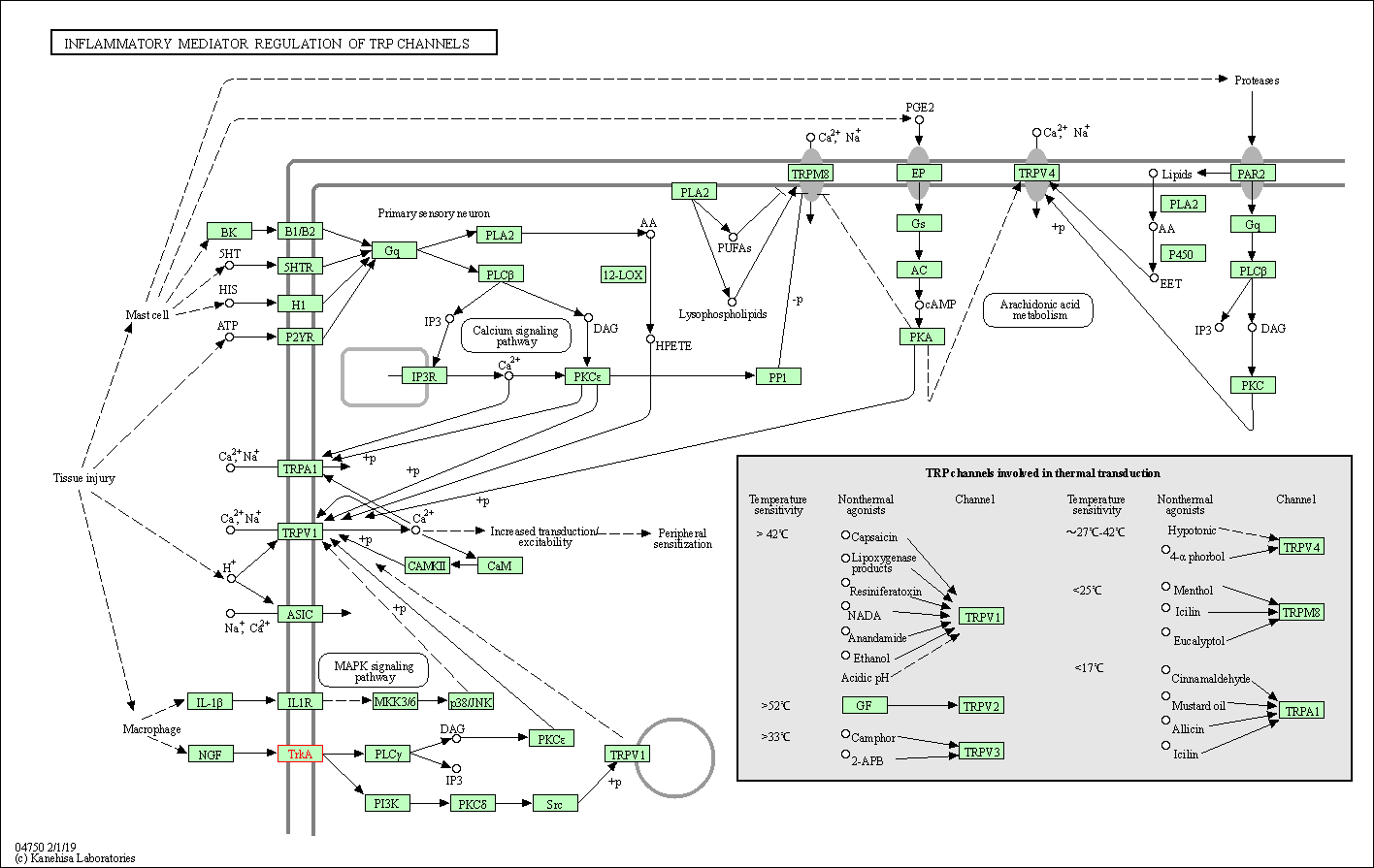
| Inflammatory mediator regulation of TRP channels | hsa04750 | Affiliated Target |

|
| Class: Organismal Systems => Sensory system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 41 | Degree centrality | 4.40E-03 | Betweenness centrality | 2.17E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.47E-01 | Radiality | 1.43E+01 | Clustering coefficient | 1.12E-01 |
| Neighborhood connectivity | 3.14E+01 | Topological coefficient | 5.60E-02 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Drug Resistance Mutation (DRM) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 9 KEGG Pathways | + | ||||
| 1 | MAPK signaling pathway | |||||
| 2 | Endocytosis | |||||
| 3 | Apoptosis | |||||
| 4 | Neurotrophin signaling pathway | |||||
| 5 | Inflammatory mediator regulation of TRP channels | |||||
| 6 | Pathways in cancer | |||||
| 7 | Transcriptional misregulation in cancer | |||||
| 8 | Thyroid cancer | |||||
| 9 | Central carbon metabolism in cancer | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | IL2 Signaling Pathway | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | Neurotrophic factor-mediated Trk receptor signaling | |||||
| Reactome | [+] 4 Reactome Pathways | + | ||||
| 1 | Frs2-mediated activation | |||||
| 2 | ARMS-mediated activation | |||||
| 3 | NGF-independant TRKA activation | |||||
| 4 | PI3K/AKT activation | |||||
| WikiPathways | [+] 4 WikiPathways | + | ||||
| 1 | MAPK Signaling Pathway | |||||
| 2 | BDNF signaling pathway | |||||
| 3 | Integrated Pancreatic Cancer Pathway | |||||
| 4 | NGF signalling via TRKA from the plasma membrane | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | |||||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 4 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 218213 | |||||
| REF 5 | ClinicalTrials.gov (NCT01960010) A Safety and Efficacy Study of MIM-D3 Ophthalmic Solution for the Treatment of Dry Eye. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT01808157) A Study to Evaluate the Efficacy, Safety and Tolerability of CT327 in Atopic Dermatitis. U.S. National Institutes of Health. | |||||
| REF 7 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 8 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800040094) | |||||
| REF 9 | ClinicalTrials.gov (NCT02279433) A First-in-human Study to Evaluate the Safety, Tolerability and Pharmacokinetics of DS-6051b. U.S. National Institutes of Health. | |||||
| REF 10 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800038879) | |||||
| REF 11 | ClinicalTrials.gov (NCT03556228) Oral TrkA Inhibitor VMD-928 for Treatment of Advanced Adult Solid Tumors or Lymphoma. U.S. National Institutes of Health. | |||||
| REF 12 | Tropomyosin receptor kinase inhibitors: an updated patent review for 2010-2016 - Part II.Expert Opin Ther Pat. 2017 Jul;27(7):831-849. | |||||
| REF 13 | Tropomyosin receptor kinase inhibitors: an updated patent review for 2010-2016 - Part I.Expert Opin Ther Pat. 2017 Jun;27(6):733-751. | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800028850) | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800037108) | |||||
| REF 16 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 17 | Safety and efficacy of MIM-D3 ophthalmic solutions in a randomized, placebo-controlled Phase 2 clinical trial in patients with dry eye. Clin Ophthalmol. 2013; 7: 1275-1285. | |||||
| REF 18 | Topical TrkA Kinase Inhibitor CT327 is an Effective, Novel Therapy for the Treatment of Pruritus due to Psoriasis: Results from Experimental Studies, and Efficacy and Safety of CT327 in a Phase 2b Clinical Trial in Patients with Psoriasis. Acta Derm Venereol. 2015 May;95(5):542-8. | |||||
| REF 19 | National Cancer Institute Drug Dictionary (drug id 766123). | |||||
| REF 20 | National Cancer Institute Drug Dictionary (drug id 747694). | |||||
| REF 21 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | |||||
| REF 22 | Preclinical trail of FX007 for treating osteoarthritis. Flexion Therapeutics Inc. | |||||
| REF 23 | Mixed-lineage kinase 1 and mixed-lineage kinase 3 subtype-selective dihydronaphthyl[3,4-a]pyrrolo[3,4-c]carbazole-5-ones: optimization, mixed-linea... J Med Chem. 2008 Sep 25;51(18):5680-9. | |||||
| REF 24 | Discovery and in vitro evaluation of potent TrkA kinase inhibitors: oxindole and aza-oxindoles. Bioorg Med Chem Lett. 2004 Feb 23;14(4):953-7. | |||||
| REF 25 | Synthesis, modeling, and in vitro activity of (3'S)-epi-K-252a analogues. Elucidating the stereochemical requirements of the 3'-sugar alcohol on tr... J Med Chem. 2005 Jun 2;48(11):3776-83. | |||||
| REF 26 | Antitumor Activity and Safety of the Pan-TRK, ROS1, and ALK inhibitor Entrectinib (RXDX-101): Combined Results from Two Phase I Trials | |||||
| REF 27 | Molecular Characteristics of Repotrectinib That Enable Potent Inhibition of TRK Fusion Proteins and Resistant Mutations. Mol Cancer Ther. 2021 Dec;20(12):2446-2456. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

