Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T38301
(Former ID: TTDS00421)
|
|||||
| Target Name |
Ribonucleoside-diphosphate reductase M2 (RRM2)
|
|||||
| Synonyms |
Ribonucleotide reductase small subunit; Ribonucleotide reductase small chain; Ribonucleoside-diphosphate reductase subunit M2; RR2
Click to Show/Hide
|
|||||
| Gene Name |
RRM2
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Myeloproliferative neoplasm [ICD-11: 2A20] | |||||
| 2 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
Catalyzes the biosynthesis of deoxyribonucleotides from the corresponding ribonucleotides. Inhibits Wnt signaling. Provides the precursors necessary for DNA synthesis.
Click to Show/Hide
|
|||||
| BioChemical Class |
CH/CH(2) oxidoreductase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 1.17.4.1
|
|||||
| Sequence |
MLSLRVPLAPITDPQQLQLSPLKGLSLVDKENTPPALSGTRVLASKTARRIFQEPTEPKT
KAAAPGVEDEPLLRENPRRFVIFPIEYHDIWQMYKKAEASFWTAEEVDLSKDIQHWESLK PEERYFISHVLAFFAASDGIVNENLVERFSQEVQITEARCFYGFQIAMENIHSEMYSLLI DTYIKDPKEREFLFNAIETMPCVKKKADWALRWIGDKEATYGERVVAFAAVEGIFFSGSF ASIFWLKKRGLMPGLTFSNELISRDEGLHCDFACLMFKHLVHKPSEERVREIIINAVRIE QEFLTEALPVKLIGMNCTLMKQYIEFVADRLMLELGFSKVFRVENPFDFMENISLEGKTN FFEKRVGEYQRMGVMSSPTENSFTLDADF Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 2 Approved Drugs | + | ||||
| 1 | Gemcitabine | Drug Info | Approved | Solid tumour/cancer | [2], [3] | |
| 2 | Hydroxyurea | Drug Info | Approved | Chronic myelogenous leukaemia | [4], [5] | |
| Clinical Trial Drug(s) | [+] 4 Clinical Trial Drugs | + | ||||
| 1 | CO-101 | Drug Info | Phase 2 | Pancreatic cancer | [6] | |
| 2 | Triapine | Drug Info | Phase 2 | Nerve injury | [7] | |
| 3 | CALAA-01 | Drug Info | Phase 1 | Solid tumour/cancer | [8] | |
| 4 | LY-2334737 | Drug Info | Phase 1 | Solid tumour/cancer | [9] | |
| Discontinued Drug(s) | [+] 2 Discontinued Drugs | + | ||||
| 1 | Gallium maltolate | Drug Info | Discontinued in Phase 2 | Human immunodeficiency virus infection | [10] | |
| 2 | MDL 101,731 | Drug Info | Discontinued in Phase 2 | Gastric adenocarcinoma | [11] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | NKP-46 | Drug Info | Preclinical | Solid tumour/cancer | [12] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Modulator | [+] 6 Modulator drugs | + | ||||
| 1 | Gemcitabine | Drug Info | [1] | |||
| 2 | Hydroxyurea | Drug Info | [1] | |||
| 3 | Triapine | Drug Info | [7] | |||
| 4 | CALAA-01 | Drug Info | [14] | |||
| 5 | LY-2334737 | Drug Info | [15], [16] | |||
| 6 | MDL 101,731 | Drug Info | [18] | |||
| Inhibitor | [+] 1 Inhibitor drugs | + | ||||
| 1 | CO-101 | Drug Info | [13] | |||
| Binder | [+] 1 Binder drugs | + | ||||
| 1 | Gallium maltolate | Drug Info | [17] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
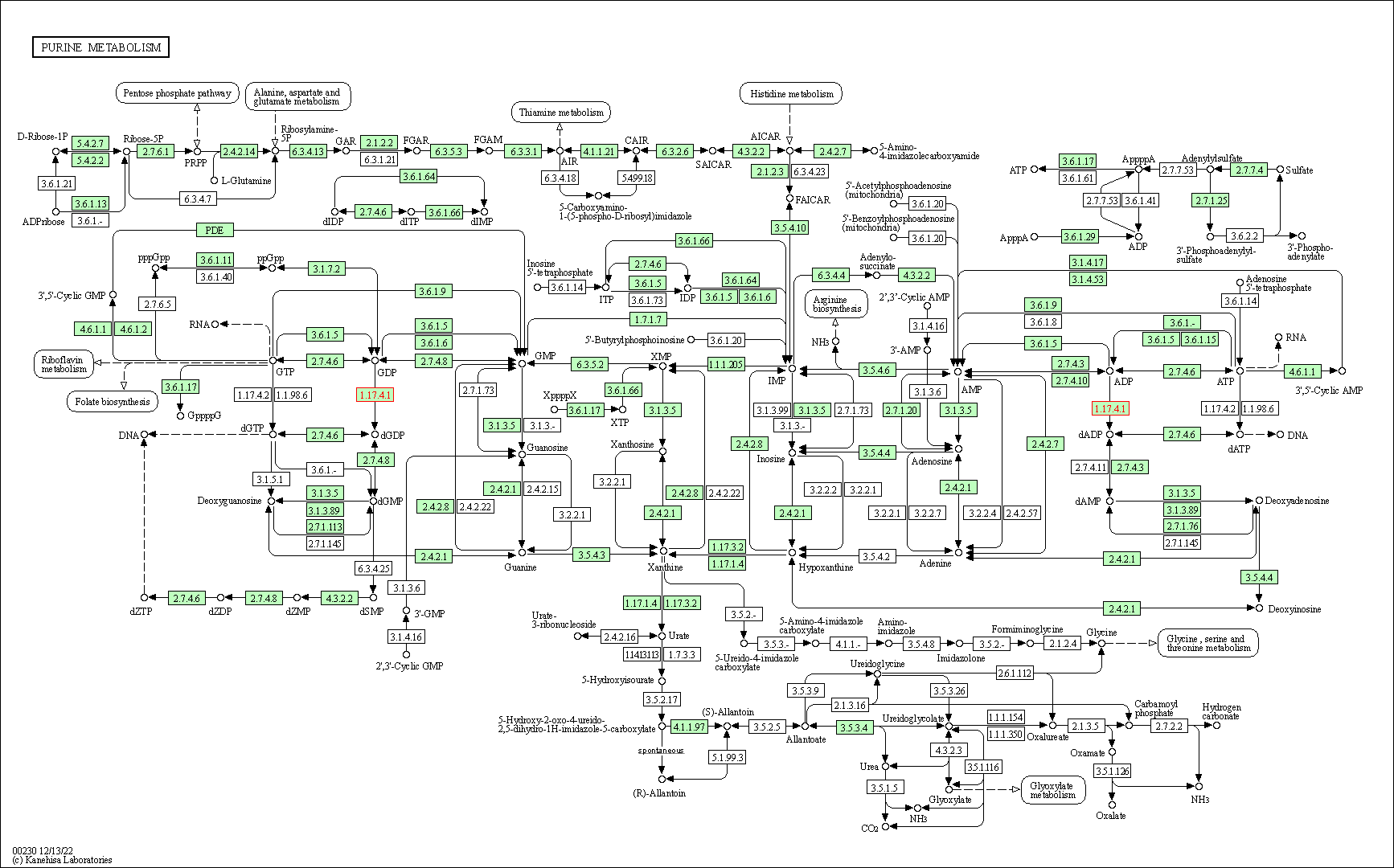
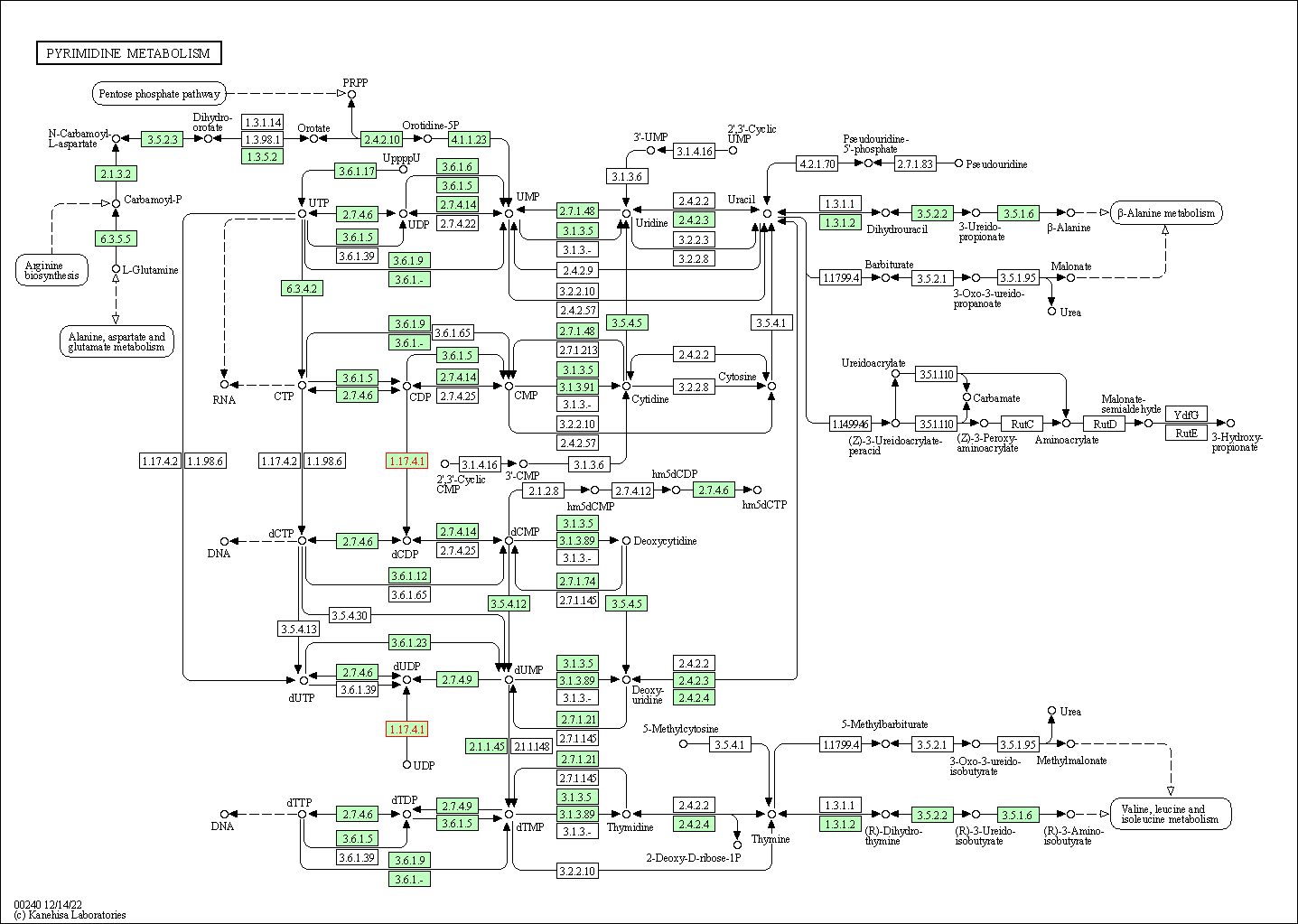
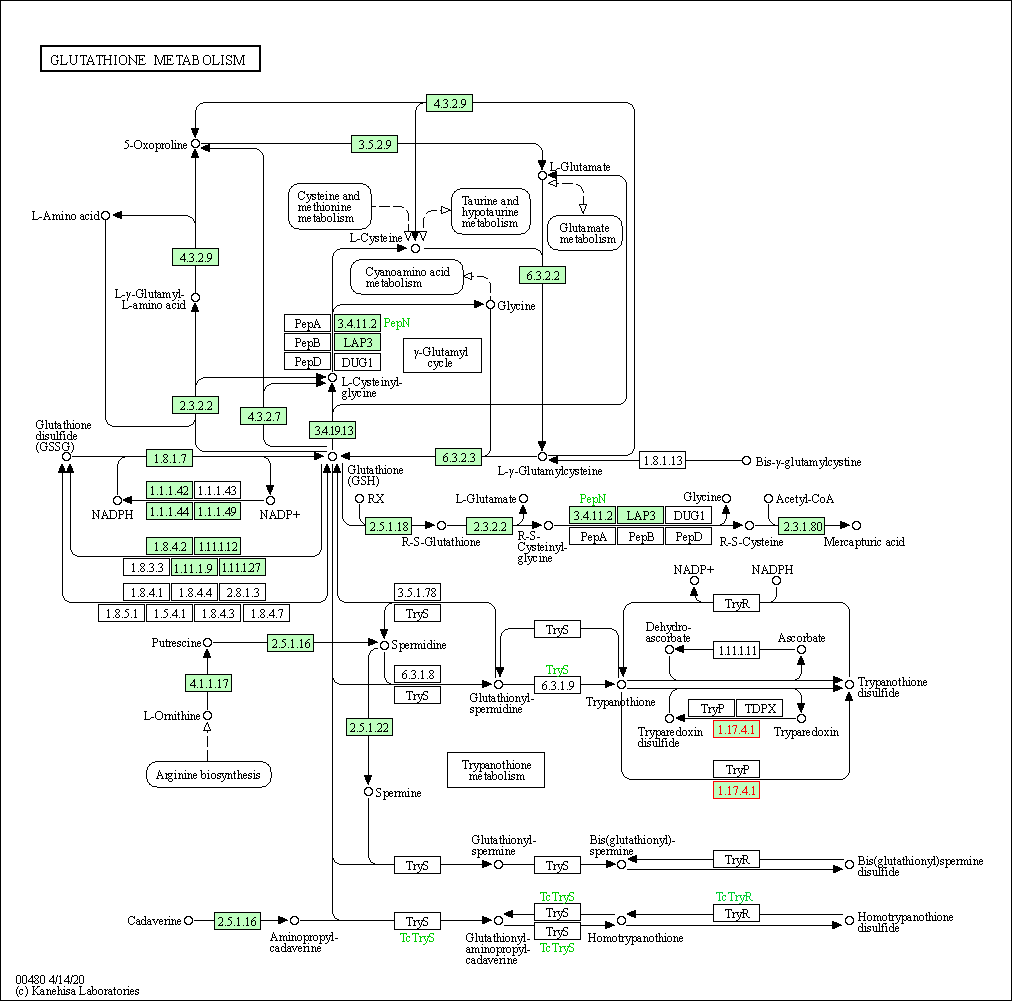
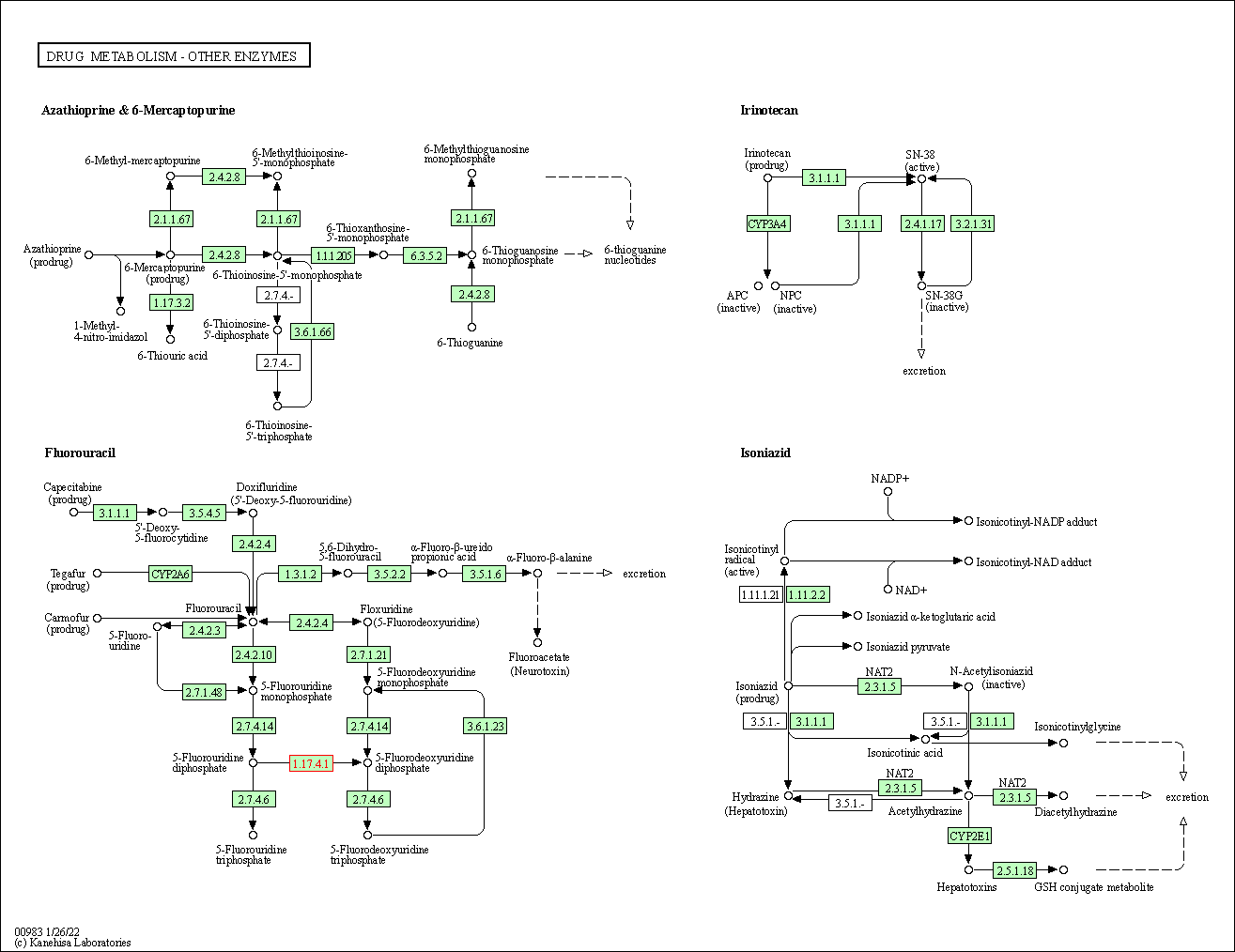
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Purine metabolism | hsa00230 | Affiliated Target |

|
| Class: Metabolism => Nucleotide metabolism | Pathway Hierarchy | ||
| Pyrimidine metabolism | hsa00240 | Affiliated Target |

|
| Class: Metabolism => Nucleotide metabolism | Pathway Hierarchy | ||
| Glutathione metabolism | hsa00480 | Affiliated Target |

|
| Class: Metabolism => Metabolism of other amino acids | Pathway Hierarchy | ||
| Drug metabolism - other enzymes | hsa00983 | Affiliated Target |

|
| Class: Metabolism => Xenobiotics biodegradation and metabolism | Pathway Hierarchy | ||
| p53 signaling pathway | hsa04115 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Degree | 30 | Degree centrality | 3.22E-03 | Betweenness centrality | 1.30E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.22E-01 | Radiality | 1.39E+01 | Clustering coefficient | 4.55E-01 |
| Neighborhood connectivity | 4.62E+01 | Topological coefficient | 1.20E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4793). | |||||
| REF 3 | Emerging drugs in cutaneous T cell lymphoma. Expert Opin Emerg Drugs. 2008 Jun;13(2):345-61. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6822). | |||||
| REF 5 | Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psori... J Am Acad Dermatol. 2009 Sep;61(3):451-85. | |||||
| REF 6 | ClinicalTrials.gov (NCT01233375) Study to Evaluate Efficacy of CO-1.01 as Second Line Therapy for Gemcitabine-Refractory Stage IV Pancreatic Adenocarcinoma. U.S. National Institutes of Health. | |||||
| REF 7 | PAN-811 inhibits oxidative stress-induced cell death of human Alzheimer's disease-derived and age-matched olfactory neuroepithelial cells via suppression of intracellular reactive oxygen species. J Alzheimers Dis. 2009;17(3):611-9. | |||||
| REF 8 | ClinicalTrials.gov (NCT00689065) Safety Study of CALAA-01 to Treat Solid Tumor Cancers. U.S. National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT01648764) A Study of LY2334737 in Participants With Cancer That is Advanced and/or Has Spread. U.S. National Institutes of Health. | |||||
| REF 10 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800014315) | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002544) | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007583) | |||||
| REF 13 | Cellular pharmacology of gemcitabine. Ann Oncol. 2006 May;17 Suppl 5:v7-12. | |||||
| REF 14 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 15 | Phase I study of oral gemcitabine prodrug (LY2334737) in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013 Jun;71(6):1645-55. | |||||
| REF 16 | Role of gemcitabine in cancer therapy.Future Oncol.2005 Feb;1(1):7-17. | |||||
| REF 17 | Gallium maltolate is a promising chemotherapeutic agent for the treatment of hepatocellular carcinoma. Anticancer Res. 2006 May-Jun;26(3A):1739-43. | |||||
| REF 18 | Tezacitabine Hoechst Marion Roussel.Curr Opin Investig Drugs.2000 Sep;1(1):135-40. | |||||
| REF 19 | Phase I and pharmacokinetic study of the oral tris-(8-quinolinolato)gallium(III) complex (FFC11, KP46) in patients with solid tumors - a study of the CESAR Central European Society for Anticancer Drug Research - EWIV. J Clin Oncol (Meeting Abstracts) June 2005 vol. 23 no. 16_suppl 3205. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

