Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T00239
(Former ID: TTDR00086)
|
|||||
| Target Name |
Interleukin-4 (IL4)
|
|||||
| Synonyms |
Pitrakinra; Lymphocyte stimulatory factor 1; IL-4; Binetrakin; BSF-1; B-cell stimulatory factor 1
Click to Show/Hide
|
|||||
| Gene Name |
IL4
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Pulmonary eosinophilia [ICD-11: CB02] | |||||
| Function |
It is a costimulator of DNA-synthesis. It induces the expression of class II MHC molecules on resting B-cells. It enhances both secretion and cell surface expression of IgE and IgG1. It also regulates the expression of the low affinity Fc receptor for IgE (CD23) on both lymphocytes and monocytes. Positively regulates IL31RA expression in macrophages. Stimulates autophagy in dendritic cells by interfering with mTORC1 signaling and through the induction of RUFY4. Participates in at least several B-cell activation processes as well as of other cell types.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine: interleukin
|
|||||
| UniProt ID | ||||||
| Sequence |
MGLTSQLLPPLFFLLACAGNFVHGHKCDITLQEIIKTLNSLTEQKTLCTELTVTDIFAAS
KNTTEKETFCRAATVLRQFYSHHEKDTRCLGATAQQFHRHKQLIRFLKRLDRNLWGLAGL NSCPVKEANQSTLENFLERLKTIMREKYSKCSS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A02929 | |||||
| HIT2.0 ID | T50V79 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 8 Clinical Trial Drugs | + | ||||
| 1 | Dexpramipexole | Drug Info | Phase 3 | Eosinophilic asthma | [2] | |
| 2 | Pascolizumab | Drug Info | Phase 2 | Asthma | [3], [4], [5] | |
| 3 | PEGylated pitrakinra | Drug Info | Phase 2 | Asthma | [6], [7] | |
| 4 | Romilkimab | Drug Info | Phase 2 | Idiopathic pulmonary fibrosis | [8] | |
| 5 | SAR-156597 | Drug Info | Phase 2 | Pulmonary fibrosis | [9] | |
| 6 | Tepoxalin | Drug Info | Phase 2 | Asthma | [10] | |
| 7 | PF-07264660 | Drug Info | Phase 1 | Atopic dermatitis | [11] | |
| 8 | PF-07275315 | Drug Info | Phase 1 | Atopic dermatitis | [12] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 4 Inhibitor drugs | + | ||||
| 1 | Dexpramipexole | Drug Info | [13] | |||
| 2 | IL-4R | Drug Info | [1] | |||
| 3 | Romilkimab | Drug Info | [15] | |||
| 4 | Tepoxalin | Drug Info | [17] | |||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | Pascolizumab | Drug Info | [14] | |||
| 2 | PEGylated pitrakinra | Drug Info | [14] | |||
| 3 | SAR-156597 | Drug Info | [16] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| IL-17 signaling pathway | hsa04657 | Affiliated Target |
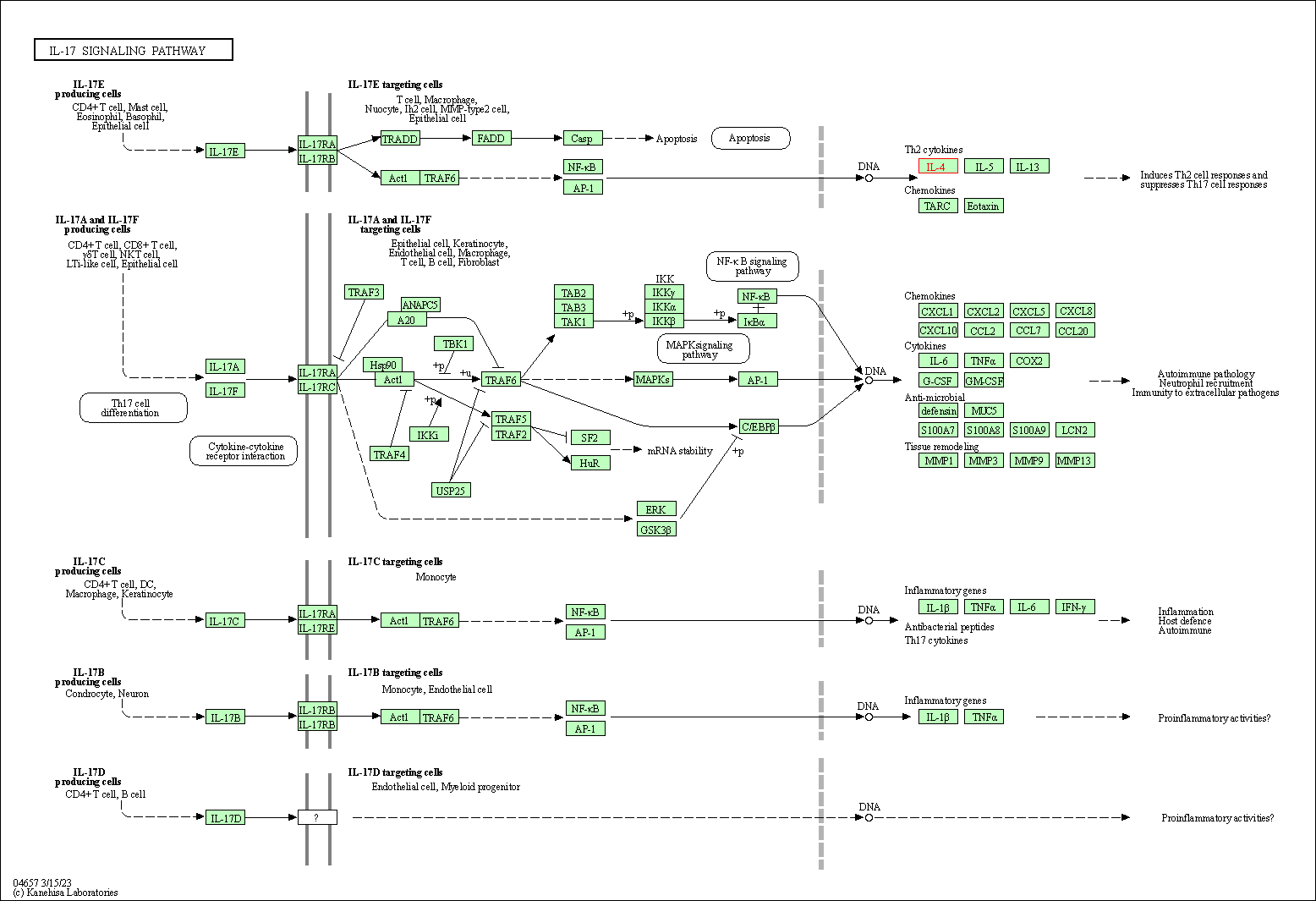
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Th1 and Th2 cell differentiation | hsa04658 | Affiliated Target |
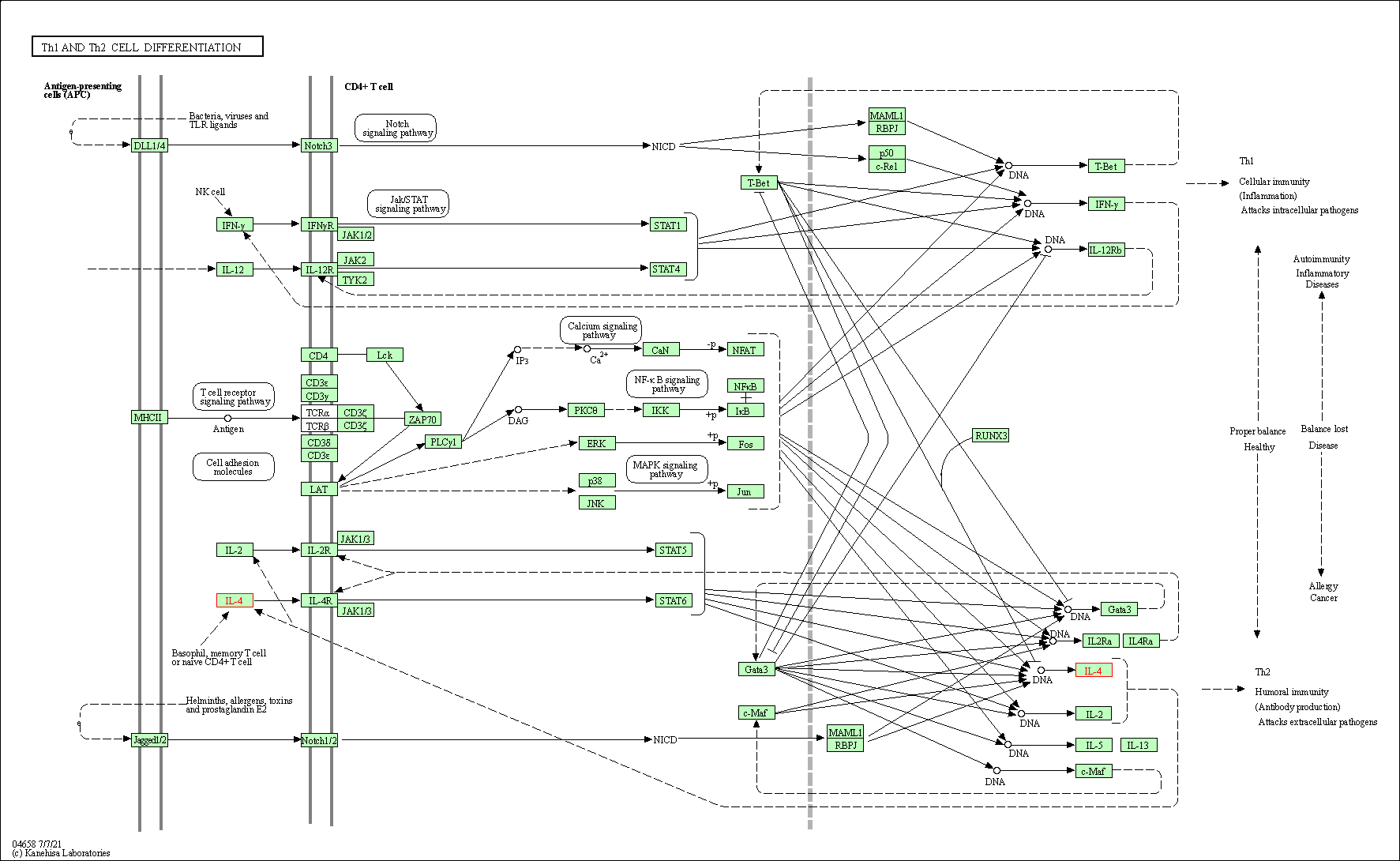
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Th17 cell differentiation | hsa04659 | Affiliated Target |
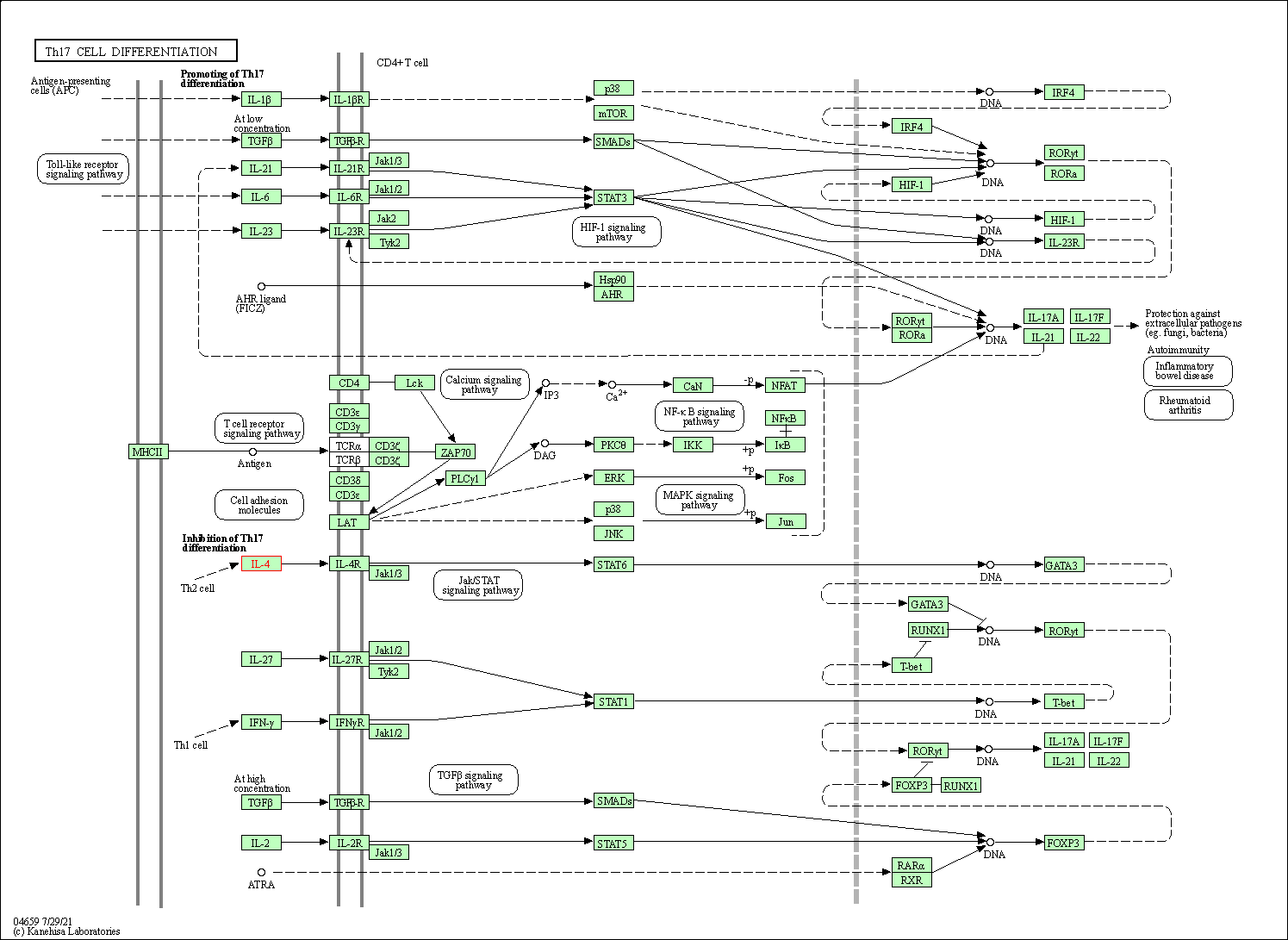
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| T cell receptor signaling pathway | hsa04660 | Affiliated Target |
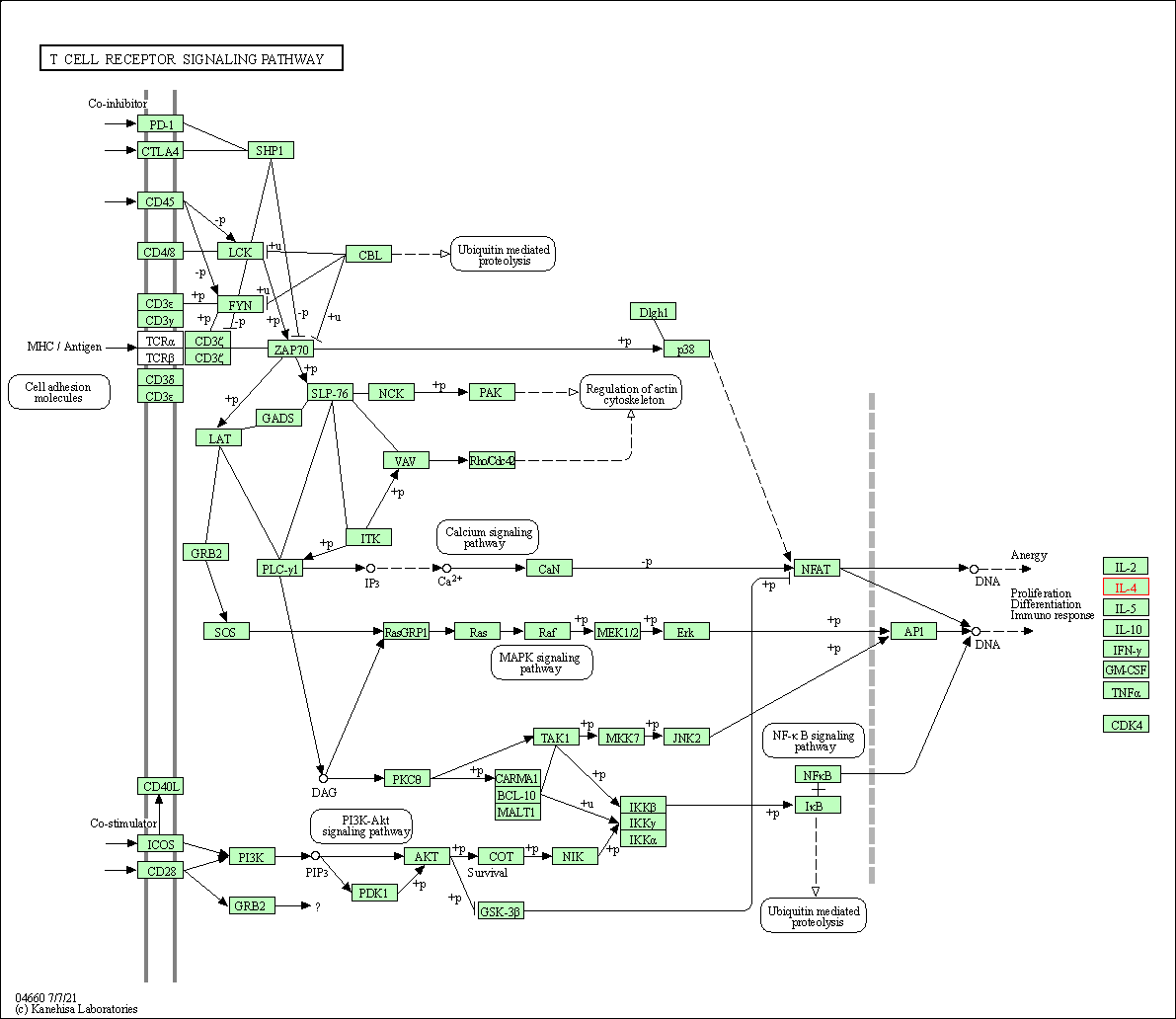
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Fc epsilon RI signaling pathway | hsa04664 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Intestinal immune network for IgA production | hsa04672 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 25 | Degree centrality | 2.69E-03 | Betweenness centrality | 4.50E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.36E-01 | Radiality | 1.41E+01 | Clustering coefficient | 2.57E-01 |
| Neighborhood connectivity | 3.53E+01 | Topological coefficient | 8.69E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol. 2001 Jun;107(6):963-70. | |||||
| REF 2 | ClinicalTrials.gov (NCT05763121) A Randomized, Double-blind, Placebo-controlled, Parallel-group Study to Assess the Efficacy, Safety, and Tolerability of Dexpramipexole Administered Orally for 52 Weeks in Participants With Severe Eosinophilic Asthma. U.S.National Institutes of Health. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7786). | |||||
| REF 4 | ClinicalTrials.gov (NCT00024544) Pilot Study in Patients With Symptomatic Steroid-Naive Asthma. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT01638520) Safety and Efficacy of Blocking IL-4 With Pascolizumab in Patients Receiving Standard Therapy for Pulmonary Tuberculosis | |||||
| REF 6 | ClinicalTrials.gov (NCT00801853) A Study of the Treatment-Sparing Effects of AEROVANT AER 001 Inhalation Powder in Asthma Patients, AEROTRIAL | |||||
| REF 7 | ClinicalTrials.gov (NCT00676884) A Phase 2a Study to Investigate the Effects of Repeated Administration of AeroDerm in Subjects With Atopic Eczema | |||||
| REF 8 | ClinicalTrials.gov (NCT02345070) Efficacy and Safety of SAR156597 in the Treatment of Idiopathic Pulmonary Fibrosis (ESTAIR). U.S. National Institutes of Health. | |||||
| REF 9 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800034106) | |||||
| REF 10 | Effects of tepoxalin, a dual inhibitor of cyclooxygenase/5-lipoxygenase, on events associated with NSAID-induced gastrointestinal inflammation. Prostaglandins Leukot Essent Fatty Acids. 1997 Jun;56(6):417-23. | |||||
| REF 11 | ClinicalTrials.gov (NCT05496738) A PHASE 1, RANDOMIZED, DOUBLE-BLIND, SPONSOR-OPEN, PLACEBO-CONTROLLED, DOSE-ESCALATING STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF SINGLE AND MULTIPLE INTRAVENOUS AND SUBCUTANEOUS DOSES OF PF-07264660 IN HEALTHY PARTICIPANTS. U.S.National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT05411588) A PHASE 1, RANDOMIZED, DOUBLE-BLIND, SPONSOR OPEN, PLACEBO-CONTROLLED, DOSE ESCALATING STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF SINGLE AND MULTIPLE INTRAVENOUS AND SUBCUTANEOUS DOSES OF PF-07275315 IN HEALTHY PARTICIPANTS. U.S.National Institutes of Health. | |||||
| REF 13 | Therapeutic strategies for eosinophilic dermatoses. Curr Opin Pharmacol. 2019 Jun;46:29-33. | |||||
| REF 14 | The potential of biologics for the treatment of asthma. Nat Rev Drug Discov. 2012 Dec;11(12):958-72. | |||||
| REF 15 | A randomised, double-blind, placebo-controlled, 24-week, phase II, proof-of-concept study of romilkimab (SAR156597) in early diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2020 Dec;79(12):1600-1607. | |||||
| REF 16 | Bispecific antibodies rise again. Nat Rev Drug Discov. 2014 Nov;13(11):799-801. | |||||
| REF 17 | Therapeutic interference with interferon-gamma (IFN-gamma) and soluble IL-4 receptor (sIL-4R) in allergic diseases. Behring Inst Mitt. 1995 Jun;(96):118-30. | |||||
| REF 18 | Clinical pipeline report, company report or official report of Pfizer | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

