Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DP2UR8
|
|||
| Drug Name |
Dexpramipexole
|
|||
| Synonyms |
Dexpramipexole; 104632-28-2; (R)-PRAMIPEXOLE; (R)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine; R-(+)-Pramipexole; (6R)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine; WI638GUS96; KNS-760704; (6R)-N6-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine; Dexpramipexole [USAN:INN]; UNII-WI638GUS96; (R)-Pramipexole;R-(+)-Pramipexole;KNS-760704; Dexpramipexole (USAN/INN); DEXPRAMIPEXOLE [INN]; SCHEMBL74780; DEXPRAMIPEXOLE [USAN]; MLS006011813; CHEMBL249420; DEXPRAMIPEXOLE [WHO-DD]; DTXSID50146624; FASDKYOPVNHBLU-SSDOTTSWSA-N; BCP12512; 2,6-Benzothiazolediamine, 4,5,6,7-tetrahydro-N6-propyl-, (6R)-; BDBM50568780; HY-17355B; AKOS005555111; CS-1196; DB15130; AC-36724; AS-56557; SMR004703492; D09886; EN300-123062; F19514; Q5268345; (6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine; (R)-4,5,6,7-TETRAHYDRO-6-(PROPYLAMINO)-BENZOTHIAZOLE-2-AMINE; 2,6-BENZOTHIAZOLEDIAMINE, 4,5,6,7-TETRAHYDRO-N6-PROPYL-, (R)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Eosinophilic asthma [ICD-11: CB02.0; ICD-9: 493] | Phase 3 | [1] | |
| Hypereosinophilic syndrome [ICD-11: 2A20.3; ICD-10: D47.5] | Phase 2 | [2] | ||
| Company |
Knopp Biosciences
|
|||
| Structure |
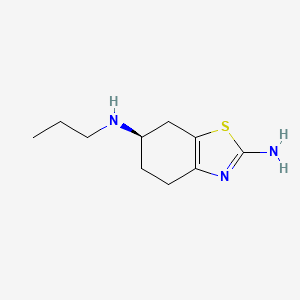 |
Download2D MOL |
||
| Formula |
C10H17N3S
|
|||
| Canonical SMILES |
CCCNC1CCC2=C(C1)SC(=N2)N
|
|||
| InChI |
InChI=1S/C10H17N3S/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8/h7,12H,2-6H2,1H3,(H2,11,13)/t7-/m1/s1
|
|||
| InChIKey |
FASDKYOPVNHBLU-SSDOTTSWSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05763121) A Randomized, Double-blind, Placebo-controlled, Parallel-group Study to Assess the Efficacy, Safety, and Tolerability of Dexpramipexole Administered Orally for 52 Weeks in Participants With Severe Eosinophilic Asthma. U.S.National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT02101138) An Open-Label, Proof-of-Concept Study to Evaluate the Safety and Efficacy of Dexpramipexole (KNS-760704) in Subjects With Hypereosinophilic Syndrome. U.S.National Institutes of Health. | |||
| REF 3 | Therapeutic strategies for eosinophilic dermatoses. Curr Opin Pharmacol. 2019 Jun;46:29-33. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

