Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0G3BI
|
|||
| Former ID |
DNC010068
|
|||
| Drug Name |
6-methyl-2-oxo-2H-chromene-3-carboxylic acid
|
|||
| Synonyms |
10242-13-4; 6-methyl-2-oxo-2H-chromene-3-carboxylic acid; 2H-1-BENZOPYRAN-3-CARBOXYLIC ACID, 6-METHYL-2-OXO-; BRN 0172386; 6-Methyl-2-oxo-2H-1-benzopyran-3-carboxylic acid; 6-methyl-2-oxochromene-3-carboxylic acid; CHEMBL577123; AC1L18EL; 5-18-08-00343 (Beilstein Handbook Reference); SCHEMBL2808135; CTK0H8591; DTXSID00145066; MolPort-007-984-853; FJICLQQBBFWGMZ-UHFFFAOYSA-N; HMS1622M11; ZINC2023765; SBB077427; BDBM50303488; AKOS002679479; MCULE-8314622881; LS-39185; VU0511228-1; 2-Oxo-6-methyl-2H-1-benzopyran-3-carboxylic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
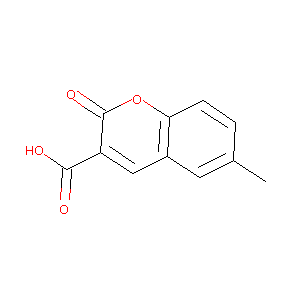 |
Download2D MOL |
||
| Formula |
C11H8O4
|
|||
| Canonical SMILES |
CC1=CC2=C(C=C1)OC(=O)C(=C2)C(=O)O
|
|||
| InChI |
1S/C11H8O4/c1-6-2-3-9-7(4-6)5-8(10(12)13)11(14)15-9/h2-5H,1H3,(H,12,13)
|
|||
| InChIKey |
FJICLQQBBFWGMZ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 10242-13-4
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem. 2010 Jan 14;53(1):335-44. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

