Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01EUF
|
||||
| Former ID |
DCL000003
|
||||
| Drug Name |
BMS 275291
|
||||
| Synonyms |
D 2163; N-((2S)-2-Mercapto-1-oxo-4-(3,4,4-trimethyl-2,5-dioxo-1-imidazolidinyl)butyl)-L-leucyl-N,3-dimethyl-L-valinamide; (2S)-N-[(2S)-3,3-dimethyl-2-(methylamino)butanoyl]-4-methyl-2-[[(2S)-2-sulfanyl-4-(3,4,4-trimethyl-2,5-dioxoimidazolidin-1-yl)butanoyl]amino]pentanamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Non-small-cell lung cancer; Hormone-refractory prostate cancer; Kaposi's sarcoma [ICD9: 140-229, 162, 176, 185, 204.0; ICD10:C33, C33-C34, C34, C46, C61, C91.0] | Discontinued in Phase 3 | [536608] | ||
| Therapeutic Class |
Anticancer Agents
|
||||
| Company |
Bristol Myers Squibb; Celltech Group
|
||||
| Structure |
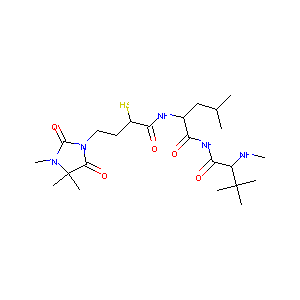
|
Download2D MOL |
|||
| Formula |
C23H41N5O5S
|
||||
| Canonical SMILES |
CC(C)CC(C(=O)NC(=O)C(C(C)(C)C)NC)NC(=O)C(CCN1C(=O)C(N(C<br />1=O)C)(C)C)S
|
||||
| InChI |
1S/C23H41N5O5S/c1-13(2)12-14(17(29)26-19(31)16(24-8)22(3,4)5)25-18(30)15(34)10-11-28-20(32)23(6,7)27(9)21(28)33/h13-16,24,34H,10-12H2,1-9H3,(H,25,30)(H,26,29,31)/t14-,15-,16+/m0/s1
|
||||
| InChIKey |
BWLOJMZLTBBDHA-HRCADAONSA-N
|
||||
| CAS Number |
CAS 259188-38-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | 72 kDa type IV collagenase | Target Info | Inhibitor | [528049], [536019], [536073], [536608] | |
| Interstitial collagenase | Target Info | Inhibitor | [528049], [536019], [536073], [536608] | ||
| Matrilysin | Target Info | Inhibitor | [528049], [536019], [536073], [536608] | ||
| Pathway Interaction Database | LPA receptor mediated events | ||||
| Plasma membrane estrogen receptor signaling | |||||
| Osteopontin-mediated events | |||||
| Validated transcriptional targets of AP1 family members Fra1 and Fra2 | |||||
| Angiopoietin receptor Tie2-mediated signaling | |||||
| Direct p53 effectors | |||||
| amb2 Integrin signaling | |||||
| ATF-2 transcription factor network | |||||
| FOXM1 transcription factor network | |||||
| Regulation of nuclear beta catenin signaling and target gene transcription | |||||
| Syndecan-2-mediated signaling eventsendothelinpathway:Endothelins | |||||
| Glucocorticoid receptor regulatory network | |||||
| AP-1 transcription factor network | |||||
| Syndecan-1-mediated signaling eventsajdiss_2pathway:Posttranslational regulation of adherens junction stability and dissassembly | |||||
| p75(NTR)-mediated signaling | |||||
| Syndecan-1-mediated signaling events | |||||
| Reactome | Collagen degradation | ||||
| Degradation of the extracellular matrix | |||||
| Activation of Matrix Metalloproteinases | |||||
| Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) | |||||
| EPH-ephrin mediated repulsion of cellsR-HSA-1442490:Collagen degradation | |||||
| Basigin interactions | |||||
| Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs)R-HSA-1442490:Collagen degradation | |||||
| Assembly of collagen fibrils and other multimeric structures | |||||
| WikiPathways | Activation of Matrix Metalloproteinases | ||||
| AGE/RAGE pathway | |||||
| Matrix MetalloproteinasesWP366:TGF beta Signaling Pathway | |||||
| Bladder Cancer | |||||
| Degradation of collagen | |||||
| Quercetin and Nf-kB/ AP-1 Induced Cell Apoptosis | |||||
| Oncostatin M Signaling Pathway | |||||
| Prostate Cancer | |||||
| Integrated Breast Cancer Pathway | |||||
| Integrated Cancer pathway | |||||
| Cell surface interactions at the vascular wall | |||||
| Matrix MetalloproteinasesWP399:Wnt Signaling Pathway and Pluripotency | |||||
| Matrix Metalloproteinases | |||||
| References | |||||
| Ref 528049 | Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006 Mar;6(3):227-39. | ||||
| Ref 536019 | Randomized phase II feasibility study of combining the matrix metalloproteinase inhibitor BMS-275291 with paclitaxel plus carboplatin in advanced non-small cell lung cancer. Lung Cancer. 2004 Dec;46(3):361-8. | ||||
| Ref 536073 | Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18. J Clin Oncol. 2005 Apr 20;23(12):2831-9. | ||||
| Ref 536608 | Phase 1/2 trial of BMS-275291 in patients with human immunodeficiency virus-related Kaposi sarcoma: a multicenter trial of the AIDS Malignancy Consortium. Cancer. 2008 Mar 1;112(5):1083-8. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

