Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T99189
|
|||||
| Target Name |
Sodium/bile acid cotransporter (SLC10A1)
|
|||||
| Synonyms |
Solute carrier family 10 member 1; Sodium/taurocholate cotransporting polypeptide; Na(+)/taurocholate transport protein; Na(+)/bile acid cotransporter; Cell growth-inhibiting gene 29 protein
Click to Show/Hide
|
|||||
| Gene Name |
SLC10A1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Hepatitis virus infection [ICD-11: 1E50-1E51] | |||||
| Function |
The hepatic sodium/bile acid uptake system exhibits broad substrate specificity and transports various non-bile acid organic compounds as well. It is strictly dependent on the extracellular presence of sodium.
Click to Show/Hide
|
|||||
| BioChemical Class |
Bile acid:sodium symporter (BASS) (TC 2.A.28) family
|
|||||
| UniProt ID | ||||||
| Sequence |
MEAHNASAPFNFTLPPNFGKRPTDLALSVILVFMLFFIMLSLGCTMEFSKIKAHLWKPKG
LAIALVAQYGIMPLTAFVLGKVFRLKNIEALAILVCGCSPGGNLSNVFSLAMKGDMNLSI VMTTCSTFCALGMMPLLLYIYSRGIYDGDLKDKVPYKGIVISLVLVLIPCTIGIVLKSKR PQYMRYVIKGGMIIILLCSVAVTVLSAINVGKSIMFAMTPLLIATSSLMPFIGFLLGYVL SALFCLNGRCRRTVSMETGCQNVQLCSTILNVAFPPEVIGPLFFFPLLYMIFQLGEGLLL IAIFWCYEKFKTPKDKTKMIYTAATTEETIPGALGNGTYKGEDCSPCTA Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Bulevirtide | Drug Info | Approved | Hepatitis D virus infection | [2] | |
| Clinical Trial Drug(s) | [+] 1 Clinical Trial Drugs | + | ||||
| 1 | LUM001 | Drug Info | Phase 2 | Primary biliary cirrhosis | [3] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Inhibitor | [+] 2 Inhibitor drugs | + | ||||
| 1 | Bulevirtide | Drug Info | [4] | |||
| 2 | LUM001 | Drug Info | [1] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Cholesterol | Ligand Info | |||||
| Structure Description | Structure of the human sodium/bile acid cotransporter (NTCP) in complex with Fab and nanobody | PDB:7ZYI | ||||
| Method | Electron microscopy | Resolution | 2.88 Å | Mutation | No | [5] |
| PDB Sequence |
GKRPTDLALS
28 VILVFMLFFI38 MLSLGCTMEF48 SKIKAHLWKP58 KGLAIALVAQ68 YGIMPLTAFV 78 LGKVFRLKNI88 EALAILVCGC98 SPGGNLSNVF108 SLAMKGDMNL118 SIVMTTCSTF 128 CALGMMPLLL138 YIYSRGIYDG148 DLKDKVPYKG158 IVISLVLVLI168 PCTIGIVLKS 178 KRPQYMRYVI188 KGGMIIILLC198 SVAVTVLSAI208 NVGKSIMFAM218 TPLLIATSSL 228 MPFIGFLLGY238 VLSALFCLNG248 RCRRTVSMET258 GCQNVQLCST268 ILNVAFPPEV 278 IGPLFFFPLL288 YMIFQLGEGL298 LLIAIFWCYE308 KFK
|
|||||
|
|
THR203
4.791
SER206
3.446
ALA207
3.708
VAL210
3.547
MET215
3.550
PHE216
3.225
ALA217
3.926
MET218
3.524
PHE231
3.810
LEU235
3.605
TYR238
2.376
|
|||||
| Ligand Name: glycochenodeoxycholic acid | Ligand Info | |||||
| Structure Description | Structure of the human sodium/bile acid cotransporter (NTCP) in complex with Fab and nanobody | PDB:7ZYI | ||||
| Method | Electron microscopy | Resolution | 2.88 Å | Mutation | No | [5] |
| PDB Sequence |
GKRPTDLALS
28 VILVFMLFFI38 MLSLGCTMEF48 SKIKAHLWKP58 KGLAIALVAQ68 YGIMPLTAFV 78 LGKVFRLKNI88 EALAILVCGC98 SPGGNLSNVF108 SLAMKGDMNL118 SIVMTTCSTF 128 CALGMMPLLL138 YIYSRGIYDG148 DLKDKVPYKG158 IVISLVLVLI168 PCTIGIVLKS 178 KRPQYMRYVI188 KGGMIIILLC198 SVAVTVLSAI208 NVGKSIMFAM218 TPLLIATSSL 228 MPFIGFLLGY238 VLSALFCLNG248 RCRRTVSMET258 GCQNVQLCST268 ILNVAFPPEV 278 IGPLFFFPLL288 YMIFQLGEGL298 LLIAIFWCYE308 KFK
|
|||||
|
|
LEU27
4.614
SER28
4.948
LEU31
3.768
VAL32
4.124
MET34
3.964
LEU35
3.543
ILE38
3.532
GLY102
4.529
ASN103
3.692
LEU104
3.688
VAL107
4.545
SER162
4.842
ILE195
4.340
SER199
4.233
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Similarity Proteins
Human Pathway Affiliation
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
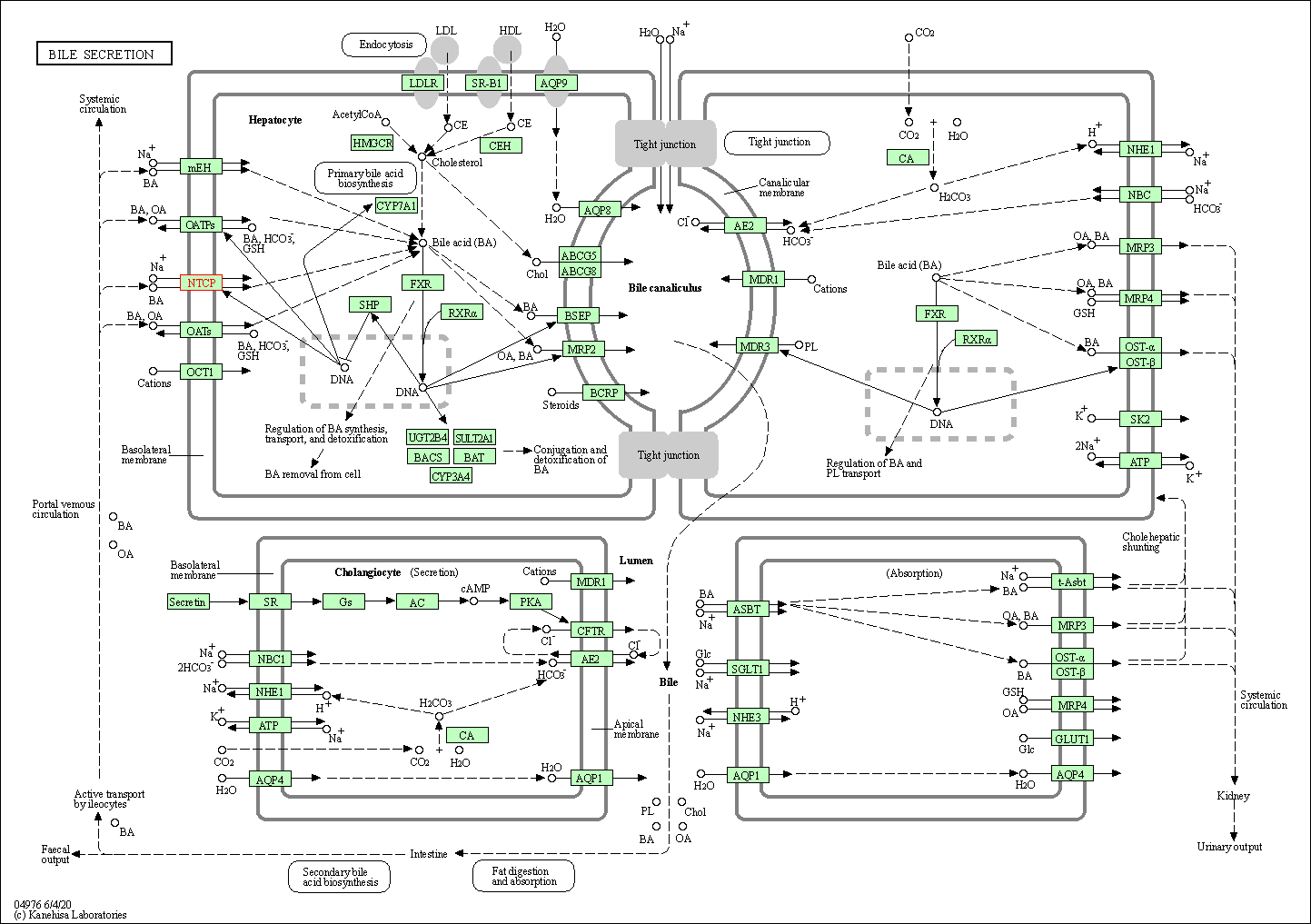
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Bile secretion | hsa04976 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 2 KEGG Pathways | + | ||||
| 1 | Bile secretion | |||||
| 2 | Hepatitis B | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 2 | ClinicalTrials.gov (NCT03852719) Phase 3 Study of Bulevirtide in Patients With CHD. U.S. National Institutes of Health. | |||||
| REF 3 | ClinicalTrials.gov (NCT02321306) An Open-label Study to Evaluate the Long-term Safety and Tolerability of LUM001 in Patients With Primary Biliary Cirrhosis. U.S. National Institutes of Health. | |||||
| REF 4 | Bulevirtide: First Approval. Drugs. 2020 Oct;80(15):1601-1605. | |||||
| REF 5 | Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

