Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T78198
(Former ID: TTDC00091)
|
|||||
| Target Name |
Purine nucleoside phosphorylase (PNP)
|
|||||
| Synonyms |
PNP; Inosine phosphorylase
Click to Show/Hide
|
|||||
| Gene Name |
PNP
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 5 Target-related Diseases | + | ||||
| 1 | Diabetes mellitus [ICD-11: 5A10] | |||||
| 2 | Gout [ICD-11: FA25] | |||||
| 3 | Psoriasis [ICD-11: EA90] | |||||
| 4 | Malignant haematopoietic neoplasm [ICD-11: 2B33] | |||||
| 5 | Mycosis fungoides [ICD-11: 2B01] | |||||
| Function |
The purine nucleoside phosphorylases catalyze the phosphorolytic breakdown of the N-glycosidic bond in the beta- (deoxy)ribonucleoside molecules, with the formation of the corresponding free purine bases and pentose-1-phosphate.
Click to Show/Hide
|
|||||
| BioChemical Class |
Pentosyltransferase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.4.2.1
|
|||||
| Sequence |
MENGYTYEDYKNTAEWLLSHTKHRPQVAIICGSGLGGLTDKLTQAQIFDYGEIPNFPRST
VPGHAGRLVFGFLNGRACVMMQGRFHMYEGYPLWKVTFPVRVFHLLGVDTLVVTNAAGGL NPKFEVGDIMLIRDHINLPGFSGQNPLRGPNDERFGDRFPAMSDAYDRTMRQRALSTWKQ MGEQRELQEGTYVMVAGPSFETVAECRVLQKLGADAVGMSTVPEVIVARHCGLRVFGFSL ITNKVIMDYESLEKANHEEVLAAGKQAAQKLEQFVSILMASIPLPDKAS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A05061 ; BADD_A06637 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 2 Clinical Trial Drugs | + | ||||
| 1 | BCX-3408 | Drug Info | Phase 2 | Plaque psoriasis | [2] | |
| 2 | Forodesine | Drug Info | Phase 1/2 | B-cell acute lymphoblastic leukaemia | [3], [4] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | CI-972 | Drug Info | Discontinued in Phase 1 | Rheumatoid arthritis | [5] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Inhibitor | [+] 33 Inhibitor drugs | + | ||||
| 1 | 2'3'-Dideoxyinosine | Drug Info | [6] | |||
| 2 | BCX-3408 | Drug Info | [1], [7], [8] | |||
| 3 | Forodesine | Drug Info | [9], [10], [11] | |||
| 4 | Guanosine | Drug Info | [6] | |||
| 5 | Peldesine | Drug Info | [6] | |||
| 6 | CI-972 | Drug Info | [12] | |||
| 7 | (+/-)-5'-deoxy-4'-fluoro-5'-methylthio-DADMe-ImmH | Drug Info | [13] | |||
| 8 | 2-Hydroxymethyl-Pyrrolidine-3,4-Diol | Drug Info | [14] | |||
| 9 | 3-((2-Pyrrolidine-1-yl)-ethyl)uracil | Drug Info | [15] | |||
| 10 | 3-Deoxyguanosine | Drug Info | [6] | |||
| 11 | 5'-deoxy-4'-hydroxy-5'-methylthio-DADMe-ImmH | Drug Info | [13] | |||
| 12 | 5'-Methylthio-ImmH | Drug Info | [13] | |||
| 13 | 5'-methylthio-immucillin-H | Drug Info | [15] | |||
| 14 | 5'-phenylthio-ImmH | Drug Info | [13] | |||
| 15 | 7-tert-butyl-2, 3-dihydro-3, 3-dimethyl substituted dihydrofuran 30 (DHDMBF30) | Drug Info | [16] | |||
| 16 | 8-amino-9-benzylguanine | Drug Info | [16] | |||
| 17 | 8-AMINOGUANINE | Drug Info | [17] | |||
| 18 | 8-aminoguanosine | Drug Info | [18] | |||
| 19 | 8-aza-DADMe-ImmH | Drug Info | [19] | |||
| 20 | 8-Azaguanine | Drug Info | [6] | |||
| 21 | 8-Iodo-Guanine | Drug Info | [6] | |||
| 22 | 9-(5,5-Difluoro-5-Phosphonopentyl)Guanine | Drug Info | [6], [20] | |||
| 23 | 9-Deazahypoxanthine | Drug Info | [6] | |||
| 24 | 9-Deazainosine | Drug Info | [6] | |||
| 25 | 9-DEAZAINOSINE-2',3'-O-ETHYLIDENEPHOSPHONATE | Drug Info | [21] | |||
| 26 | Aza-C-nucleosides | Drug Info | [22] | |||
| 27 | DADMe-ImmG | Drug Info | [19] | |||
| 28 | GUANOSINE-2',3'-O-ETHYLIDENEPHOSPHONATE | Drug Info | [21] | |||
| 29 | GUANOSINE-2',3'-O-METHYLIDENEPHOSPHONATE | Drug Info | [21] | |||
| 30 | Hypoxanthine | Drug Info | [6] | |||
| 31 | Immucillin-G | Drug Info | [22] | |||
| 32 | MT-Immucillin-H | Drug Info | [23] | |||
| 33 | Ribose-1-Phosphate | Drug Info | [6] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Aciclovir | Ligand Info | |||||
| Structure Description | CRYSTAL STRUCTURE OF HUMAN PNP COMPLEXED WITH ACYCLOVIR | PDB:1PWY | ||||
| Method | X-ray diffraction | Resolution | 2.80 Å | Mutation | No | [24] |
| PDB Sequence |
ENGYTYEDYK
11 NTAEWLLSHT21 KHRPQVAIIC31 GSGLGGLTDK41 LTQAQIFDYS51 EIPNFPRSTV 61 PGHAGRLVFG71 FLNGRACVMM81 QGRFHMYEGY91 PLWKVTFPVR101 VFHLLGVDTL 111 VVTNAAGGLN121 PKFEVGDIML131 IRDHINLPGF141 SGQNPLRGPN151 DERFGDRFPA 161 MSDAYDRTMR171 QRALSTWKQM181 GEQRELQEGT191 YVMVAGPSFE201 TVAECRVLQK 211 LGADAVGMST221 VPEVIVARHC231 GLRVFGFSLI241 TNKVIMDYES251 LEKANHEEVL 261 AAGKQAAQKL271 EQFVSILMAS281 IPLPDKAS
|
|||||
|
|
||||||
| Ligand Name: Didanosine | Ligand Info | |||||
| Structure Description | Structure of human PNP complexed with DDI | PDB:1V3Q | ||||
| Method | X-ray diffraction | Resolution | 2.80 Å | Mutation | No | [25] |
| PDB Sequence |
ENGYTYEDYK
11 NTAEWLLSHT21 KHRPQVAIIC31 GSGLGGLTDK41 LTQAQIFDYS51 EIPNFPRSTV 61 PGHAGRLVFG71 FLNGRACVMM81 QGRFHMYEGY91 PLWKVTFPVR101 VFHLLGVDTL 111 VVTNAAGGLN121 PKFEVGDIML131 IRDHINLPGF141 SGQNPLRGPN151 DERFGDRFPA 161 MSDAYDRTMR171 QRALSTWKQM181 GEQRELQEGT191 YVMVAGPSFE201 TVAECRVLQK 211 LGADAVGMST221 VPEVIVARHC231 GLRVFGFSLI241 TNKVIMDYES251 LEKANHEEVL 261 AAGKQAAQKL271 EQFVSILMAS281 IPLPDKAS
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Purine metabolism | hsa00230 | Affiliated Target |
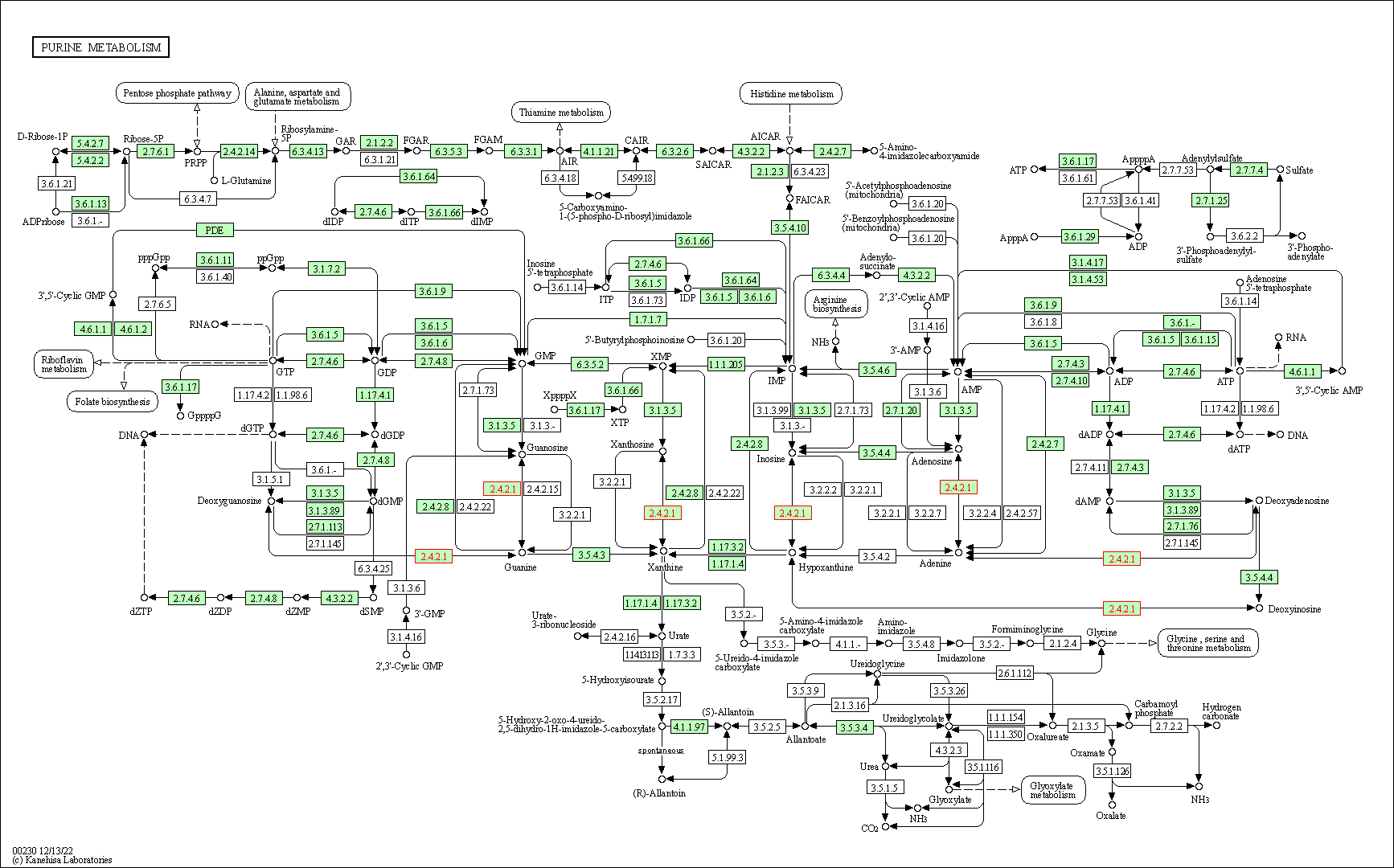
|
| Class: Metabolism => Nucleotide metabolism | Pathway Hierarchy | ||
| Nicotinate and nicotinamide metabolism | hsa00760 | Affiliated Target |
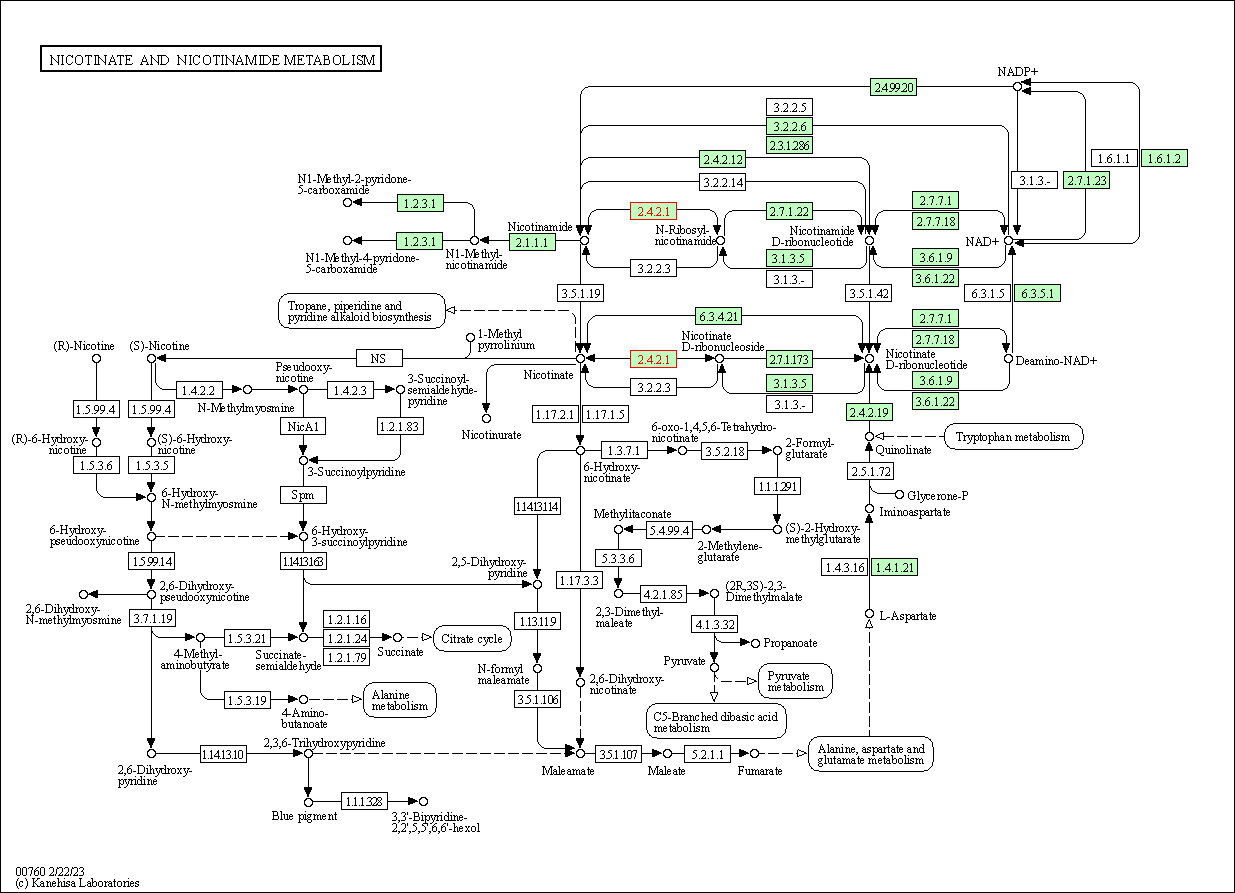
|
| Class: Metabolism => Metabolism of cofactors and vitamins | Pathway Hierarchy | ||
| Degree | 15 | Degree centrality | 1.61E-03 | Betweenness centrality | 1.01E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 1.68E-01 | Radiality | 1.26E+01 | Clustering coefficient | 1.24E-01 |
| Neighborhood connectivity | 7.53E+00 | Topological coefficient | 1.21E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | BCX-4208 (RO5092888), a Purine Nucleoside Phosphorylase (PNP) Inhibitor, Is a Novel, Potent Orally Active Anti-T-Cell and B-Cell Agent. 50th ASH Annual Meeting and Exposition. 2008. | |||||
| REF 2 | ClinicalTrials.gov (NCT01407874) A Randomized, Double-Blind, Dose-Response Study of the Safety and Uric Acid Effects of Oral Ulodesine Added to Allopurinol in Subjects With Gout and Concomitant Moderate Renal Insufficiency. U.S. National Institutes of Health. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8272). | |||||
| REF 4 | Emerging drugs in cutaneous T cell lymphoma. Expert Opin Emerg Drugs. 2008 Jun;13(2):345-61. | |||||
| REF 5 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002367) | |||||
| REF 6 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 7 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 8 | Azetidine based transition state analogue inhibitors of N-ribosyl hydrolases and phosphorylases. J Med Chem. 2008 Feb 28;51(4):948-56. | |||||
| REF 9 | Forodesine has high antitumor activity in chronic lymphocytic leukemia and activates p53-independent mitochondrial apoptosis by induction of p73 an... Blood. 2009 Aug 20;114(8):1563-75. | |||||
| REF 10 | Synthesis of analogs of forodesine HCl, a human purine nucleoside phosphorylase inhibitor-Part I. Bioorg Med Chem Lett. 2009 May 15;19(10):2624-6. | |||||
| REF 11 | Novel therapies for cutaneous T-cell lymphomas. Clin Lymphoma Myeloma. 2008 Dec;8 Suppl 5:S187-92. | |||||
| REF 12 | Inhibitors of human purine nucleoside phosphorylase. Synthesis of pyrrolo[3,2-d]pyrimidines, a new class of purine nucleoside phosphorylase inhibitors as potentially T-cell selective immunosuppressive agents. Description of 2,6-diamino-3,5-dihydro-7-(3-thienylmethyl)-4H-pyrrolo[3,2-d] pyrimidin-4-one. J Med Chem. 1992 May 1;35(9):1605-9. | |||||
| REF 13 | Immucillins in custom catalytic-site cavities. Bioorg Med Chem Lett. 2008 Nov 15;18(22):5900-3. | |||||
| REF 14 | DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Res. 2011 Jan;39(Database issue):D1035-41. | |||||
| REF 15 | Exploring new inhibitors of Plasmodium falciparum purine nucleoside phosphorylase. Eur J Med Chem. 2010 Nov;45(11):5140-9. | |||||
| REF 16 | Expression of human malaria parasite purine nucleoside phosphorylase in host enzyme-deficient erythrocyte culture. Enzyme characterization and identification of novel inhibitors. J Biol Chem. 1986 Sep 5;261(25):11667-73. | |||||
| REF 17 | Nucleosides. 5. Synthesis of guanine and formycin B derivatives as potential inhibitors of purine nucleoside phosphorylase. J Med Chem. 1993 Apr 16;36(8):1024-31. | |||||
| REF 18 | Differential metabolism of guanine nucleosides by human lymphoid cell lines. Proc Soc Exp Biol Med. 1985 Sep;179(4):427-31. | |||||
| REF 19 | Achieving the ultimate physiological goal in transition state analogue inhibitors for purine nucleoside phosphorylase. J Biol Chem. 2003 Aug 22;278(34):31465-8. | |||||
| REF 20 | Structural-based design and synthesis of novel 9-deazaguanine derivatives having a phosphate mimic as multi-substrate analogue inhibitors for mamma... Bioorg Med Chem. 2010 Mar 15;18(6):2275-2284. | |||||
| REF 21 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 22 | Exploring structure-activity relationships of transition state analogues of human purine nucleoside phosphorylase. J Med Chem. 2003 Jul 17;46(15):3412-23. | |||||
| REF 23 | Plasmodium falciparum purine nucleoside phosphorylase: crystal structures, immucillin inhibitors, and dual catalytic function. J Biol Chem. 2004 Apr 30;279(18):18103-6. | |||||
| REF 24 | Crystal structure of human purine nucleoside phosphorylase complexed with acyclovir. Biochem Biophys Res Commun. 2003 Aug 29;308(3):553-9. | |||||
| REF 25 | Structures of human purine nucleoside phosphorylase complexed with inosine and ddI. Biochem Biophys Res Commun. 2004 Jan 23;313(4):907-14. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

