Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T62206
(Former ID: TTDR01164)
|
|||||
| Target Name |
Proprotein convertase subtilisin/kexin type 9 (PCSK9)
|
|||||
| Synonyms |
Subtilisin/kexin-like protease PC9; Proprotein convertase 9; PC9; Neural apoptosis-regulated convertase 1; NARC1; NARC-1
Click to Show/Hide
|
|||||
| Gene Name |
PCSK9
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Hyper-lipoproteinaemia [ICD-11: 5C80] | |||||
| Function |
Crucial player in the regulation of plasma cholesterol homeostasis. Binds to low-density lipid receptor family members: low density lipoprotein receptor (LDLR), very low density lipoprotein receptor (VLDLR), apolipoprotein E receptor (LRP1/APOER) and apolipoprotein receptor 2 (LRP8/APOER2), and promotes their degradation in intracellular acidic compartments. Acts via a non-proteolytic mechanism to enhance the degradation of the hepatic LDLR through a clathrin LDLRAP1/ARH-mediated pathway. May prevent the recycling of LDLR from endosomes to the cell surface or direct it to lysosomes for degradation. Can induce ubiquitination of LDLR leading to its subsequent degradation. Inhibits intracellular degradation of APOB via the autophagosome/lysosome pathway in a LDLR-independent manner. Involved in the disposal of non-acetylated intermediates of BACE1 in the early secretory pathway. Inhibits epithelial Na(+) channel (ENaC)-mediated Na(+) absorption by reducing ENaC surface expression primarily by increasing its proteasomal degradation. Regulates neuronal apoptosis via modulation of LRP8/APOER2 levels and related anti-apoptotic signaling pathways.
Click to Show/Hide
|
|||||
| BioChemical Class |
Peptidase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.4.21.-
|
|||||
| Sequence |
MGTVSSRRSWWPLPLLLLLLLLLGPAGARAQEDEDGDYEELVLALRSEEDGLAEAPEHGT
TATFHRCAKDPWRLPGTYVVVLKEETHLSQSERTARRLQAQAARRGYLTKILHVFHGLLP GFLVKMSGDLLELALKLPHVDYIEEDSSVFAQSIPWNLERITPPRYRADEYQPPDGGSLV EVYLLDTSIQSDHREIEGRVMVTDFENVPEEDGTRFHRQASKCDSHGTHLAGVVSGRDAG VAKGASMRSLRVLNCQGKGTVSGTLIGLEFIRKSQLVQPVGPLVVLLPLAGGYSRVLNAA CQRLARAGVVLVTAAGNFRDDACLYSPASAPEVITVGATNAQDQPVTLGTLGTNFGRCVD LFAPGEDIIGASSDCSTCFVSQSGTSQAAAHVAGIAAMMLSAEPELTLAELRQRLIHFSA KDVINEAWFPEDQRVLTPNLVAALPPSTHGAGWQLFCRTVWSAHSGPTRMATAVARCAPD EELLSCSSFSRSGKRRGERMEAQGGKLVCRAHNAFGGEGVYAIARCCLLPQANCSVHTAP PAEASMGTRVHCHQQGHVLTGCSSHWEVEDLGTHKPPVLRPRGQPNQCVGHREASIHASC CHAPGLECKVKEHGIPAPQEQVTVACEEGWTLTGCSALPGTSHVLGAYAVDNTCVVRSRD VSTTGSTSEGAVTAVAICCRSRHLAQASQELQ Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T08VQQ | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 2 Approved Drugs | + | ||||
| 1 | Evolocumab | Drug Info | Approved | Heterozygous familial hypercholesterolemia | [2] | |
| 2 | REGN-727 | Drug Info | Approved | Familial hypercholesterolemia | [3], [4] | |
| Clinical Trial Drug(s) | [+] 10 Clinical Trial Drugs | + | ||||
| 1 | AMG 145 | Drug Info | Phase 3 | Hypercholesterolaemia | [5] | |
| 2 | Bococizumab | Drug Info | Phase 3 | Chronic obstructive pulmonary disease | [6], [7] | |
| 3 | LIB003 | Drug Info | Phase 3 | Hypercholesterolaemia | [8] | |
| 4 | PF-04950615 | Drug Info | Phase 3 | Hypercholesterolaemia | [9] | |
| 5 | LY3015014 | Drug Info | Phase 2 | Cardiovascular disease | [10] | |
| 6 | MK-0616 | Drug Info | Phase 2 | Hypercholesterolaemia | [11] | |
| 7 | AZD0780 | Drug Info | Phase 1 | Elevated Lipoprotein(a) | [12] | |
| 8 | NNC-0385-0434 | Drug Info | Phase 1 | Hypercholesterolaemia | [13] | |
| 9 | PCSK9 Adnectin | Drug Info | Phase 1 | Cardiovascular disease | [14] | |
| 10 | SPC5001 | Drug Info | Phase 1 | Hyperlipidaemia | [15] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 2 Modulator drugs | + | ||||
| 1 | AMG 145 | Drug Info | [18] | |||
| 2 | PCSK9 Adnectin | Drug Info | [22] | |||
| Inhibitor | [+] 4 Inhibitor drugs | + | ||||
| 1 | LIB003 | Drug Info | [8] | |||
| 2 | MK-0616 | Drug Info | [20] | |||
| 3 | NNC-0385-0434 | Drug Info | [13] | |||
| 4 | SPC5001 | Drug Info | [15] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
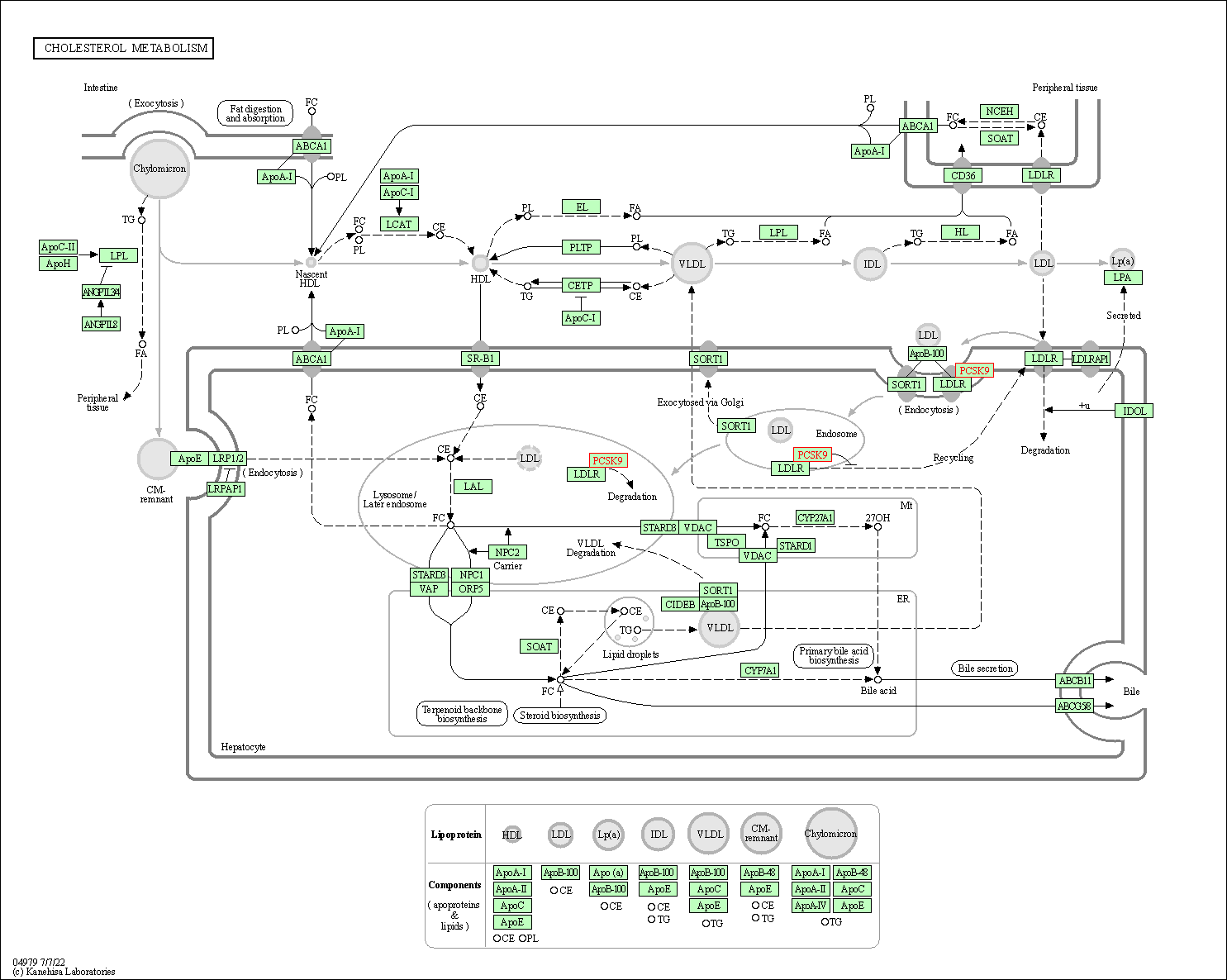
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cholesterol metabolism | hsa04979 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Degree | 4 | Degree centrality | 4.30E-04 | Betweenness centrality | 6.31E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 2.11E-01 | Radiality | 1.37E+01 | Clustering coefficient | 1.67E-01 |
| Neighborhood connectivity | 2.30E+01 | Topological coefficient | 2.78E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| WikiPathways | [+] 1 WikiPathways | + | ||||
| 1 | PCSK9-mediated LDLR degradation | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012 Jul 7;380(9836):29-36. | |||||
| REF 2 | FDA Approved Drug Products from FDA Official Website. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6744). | |||||
| REF 4 | 2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81. | |||||
| REF 5 | ClinicalTrials.gov (NCT01854918) Open Label Study of Long Term Evaluation Against LDL-C Trial-2. U.S. National Institutes of Health. | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7730). | |||||
| REF 7 | ClinicalTrials.gov (NCT02458287) Efficacy, Safety, Tolerability And Actual Use Study Of Bococizumab And An Autoinjector (Pre-Filled Pen) In Subjects With Hyperlipidemia Or Dyslipidemia. | |||||
| REF 8 | ClinicalTrials.gov (NCT05234775) Cross-Over Study to Compare the Pharmacokinetics and Pharmacodynamics of LIB003 Process 1 and Process 2 Drug Product in Subjects With or Without Statin Therapy. U.S.National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT01975376) The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT01890967) A Study of LY3015014 in Participants With High Cholesterol. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT05261126) A Phase 2b, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of MK-0616 in Adults With Hypercholesterolemia. U.S.National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT05384262) A Phase I, Randomized, Single-Blind, Placebo-controlled Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of AZD0780 Following Single and Multiple Ascending Dose Administration to Healthy Subjects With or Without Elevated LDL-C Levels. U.S.National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT04058834) A First Human Dose Trial Investigating Safety, Tolerability and Pharmacokinetics of Single Doses of NNC0385-0434 in Healthy Subjects and Patients With Hypercholesterolaemia. U.S.National Institutes of Health. | |||||
| REF 14 | Cholesterol-lowering blockbuster candidates speed into Phase III trials. Nat Rev Drug Discov. 2012 Nov;11(11):817-9. | |||||
| REF 15 | 2011 Pipeline of Santaris Pharma. | |||||
| REF 16 | Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017 May 4;376(18):1713-1722. | |||||
| REF 17 | The dyslipidaemia market. Nat Rev Drug Discov. 2014 Nov;13(11):807-8. | |||||
| REF 18 | PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015 Jan 24;385(9965):331-40. | |||||
| REF 19 | Phase 3 data for PCSK9 inhibitor wows. Nat Biotechnol. 2013 Dec;31(12):1057-8. | |||||
| REF 20 | Phase 2b Randomized Trial of the Oral PCSK9 Inhibitor MK-0616. J Am Coll Cardiol. 2023 Apr 25;81(16):1553-1564. | |||||
| REF 21 | Clinical pipeline report, company report or official report of AstraZeneca | |||||
| REF 22 | Pharmacologic profile of the Adnectin BMS-962476, a small protein biologic alternative to PCSK9 antibodies for low-density lipoprotein lowering. J Pharmacol Exp Ther. 2014 Aug;350(2):412-24. | |||||
| REF 23 | A PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces LDL cholesterol in vivo. J Lipid Res. 2011 Jan;52(1):78-86. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

