Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0B9EH
|
|||
| Former ID |
DCL000019
|
|||
| Drug Name |
Dermolastin
|
|||
| Synonyms |
Dermolastin
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Atopic dermatitis [ICD-11: EA80; ICD-10: L20; ICD-9: 691.8, 692.9] | Discontinued in Phase 2 | [1] | |
| Company |
Arriva Pharma
|
|||
| Structure |
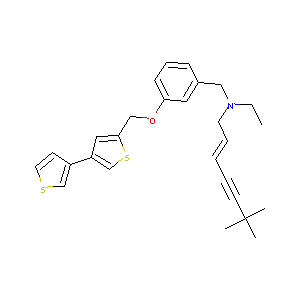 |
Download2D MOL |
||
| Formula |
C27H31NOS2
|
|||
| Canonical SMILES |
CCN(CC=CC#CC(C)(C)C)CC1=CC(=CC=C1)OCC2=CC(=CS2)C3=CSC=C3
|
|||
| InChI |
1S/C27H31NOS2/c1-5-28(14-8-6-7-13-27(2,3)4)18-22-10-9-11-25(16-22)29-19-26-17-24(21-31-26)23-12-15-30-20-23/h6,8-12,15-17,20-21H,5,14,18-19H2,1-4H3
|
|||
| InChIKey |
KIRGLCXNEVICOG-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018071) | |||
| REF 2 | Arriva-ProMetic recombinant alpha 1-antitrypsin (rAAT) moves into the clinic for dermatology applications. ProMetic Life Sciences. 2009. | |||
| REF 3 | rAAt (inhaled) Arriva/Hyland Immuno. Curr Opin Mol Ther. 2006 Feb;8(1):76-82. | |||
| REF 4 | Optimization of the bioprocessing conditions for scale-up of transient production of a heterologous protein in plants using a chemically inducible viral amplicon expression system. Biotechnol Prog. 2009 May-Jun;25(3):722-34. | |||
| REF 5 | Bioreactor strategies for improving production yield and functionality of a recombinant human protein in transgenic tobacco cell cultures. Biotechnol Bioeng. 2009 Feb 1;102(2):508-20. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

