Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07TAJ
|
|||
| Former ID |
DNC010070
|
|||
| Drug Name |
Ethyl 7-methoxy-2-oxo-2H-chromene-3-carboxylate
|
|||
| Synonyms |
ethyl 7-methoxy-2-oxo-2H-chromene-3-carboxylate; 6093-72-7; CHEMBL568385; ethyl 7-methoxy-2-oxochromene-3-carboxylate; 7-Methoxy-2-oxo-2H-chromene-3-carboxylic acid ethyl ester; MLS000554938; AC1LIZL3; Oprea1_386697; AC1Q645E; SCHEMBL1406462; CTK8D4125; CHEBI:108321; MolPort-000-258-049; ZINC499358; HMS2332G10; STK527622; BDBM50303495; AKOS002230558; MCULE-3724351908; AJ-23200; SMR000147055; TC-069558; KB-296861; AX8285479; ST50114633; 2-Oxo-7-methoxy-2H-1-benzopyran-3-carboxylic acid ethyl ester; 2H-1-Benzopyran-3-carboxylic acid,
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
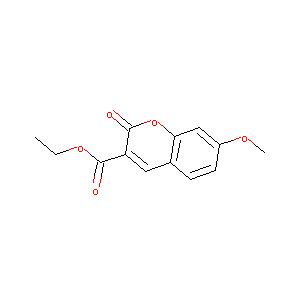 |
Download2D MOL |
||
| Formula |
C13H12O5
|
|||
| Canonical SMILES |
CCOC(=O)C1=CC2=C(C=C(C=C2)OC)OC1=O
|
|||
| InChI |
1S/C13H12O5/c1-3-17-12(14)10-6-8-4-5-9(16-2)7-11(8)18-13(10)15/h4-7H,3H2,1-2H3
|
|||
| InChIKey |
FWFVXBZXJKTVGL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 6093-72-7
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:108321
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem. 2010 Jan 14;53(1):335-44. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

