Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05TNX
|
|||
| Former ID |
DIB019863
|
|||
| Drug Name |
fluprostenol
|
|||
| Synonyms |
Fluprostenol; travoprost acid; (+)-fluprostenol; Fluprostenolum; travoprost free acid; ICI 81,008; UNII-MEH3MCE8X1; EINECS 255-029-3; 40666-16-8; CHEBI:60782; MEH3MCE8X1; 54276-17-4; NCGC00160386-01; Fluprostenol [INN:BAN]; ICI 81008; Fluprostenolum [INN-Latin]; AL-5848; rac-(5Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-{(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]but-1-en-1-yl}cyclopentyl]hept-5-enoic acid; (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]but-1-enyl]cyclopentyl]hept-5-enoic; ICI 81008; [3H](+)-fluprostenol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1], [2] | |
| Structure |
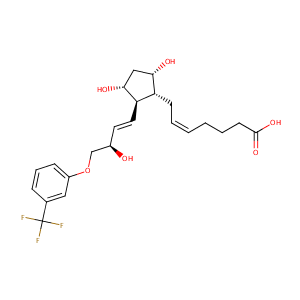 |
Download2D MOL
|
||
| Formula |
C23H29F3O6
|
|||
| Canonical SMILES |
C1C(C(C(C1O)C=CC(COC2=CC=CC(=C2)C(F)(F)F)O)CC=CCCCC(=O)O)O
|
|||
| InChI |
1S/C23H29F3O6/c24-23(25,26)15-6-5-7-17(12-15)32-14-16(27)10-11-19-18(20(28)13-21(19)29)8-3-1-2-4-9-22(30)31/h1,3,5-7,10-12,16,18-21,27-29H,2,4,8-9,13-14H2,(H,30,31)/b3-1-,11-10+/t16-,18-,19-,20+,21-/m1/s1
|
|||
| InChIKey |
WWSWYXNVCBLWNZ-QIZQQNKQSA-N
|
|||
| CAS Number |
CAS 40666-16-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7979290, 11056269, 14833691, 14959572, 26754611, 29216067, 39340820, 47216547, 47810513, 50110093, 51060530, 53786902, 85787689, 92309752, 99300666, 99302184, 104046520, 104222381, 114155262, 124892256, 124963557, 135650277, 143054778, 144205331, 163884230, 164234741, 172648729, 175269771, 178100423, 179205558, 198983226, 224677454, 226582737, 241377045, 252158156, 252455476, 252457294, 252462915, 252467640
|
|||
| ChEBI ID |
CHEBI:60782
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Prostaglandin E2 receptor EP3 (PTGER3) | Target Info | Agonist | [3] |
| Prostaglandin F2-alpha receptor (PTGFR) | Target Info | Agonist | [4], [5] | |
| Thromboxane A2 receptor (TBXA2R) | Target Info | Agonist | [3] | |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Platelet activation | ||||
| cAMP signaling pathway | ||||
| Regulation of lipolysis in adipocytes | ||||
| Pathways in cancer | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| Reactome | Prostanoid ligand receptors | |||
| G alpha (q) signalling events | ||||
| G alpha (12/13) signalling events | ||||
| Thromboxane signalling through TP receptor | ||||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Small Ligand GPCRs | ||||
| Signal amplification | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| Prostaglandin Synthesis and Regulation | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1940). | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3417). | |||
| REF 3 | The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000 Jan 17;1483(2):285-93. | |||
| REF 4 | Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997 Sep;122(2):217-24. | |||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 344). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

