Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01FQR
|
|||
| Former ID |
DCL000904
|
|||
| Drug Name |
Ocaperidone
|
|||
| Synonyms |
Ocaperidona; 129029-23-8; UNII-26HUS7139V; 3-(2-(4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidino)ethyl)-2,9-dimethyl-4H-pyrido(1,2-a)pyrimidin-4-one; 26HUS7139V; Ocaperidonum; Ocaperidonum [INN-Latin]; Ocaperidona [INN-Spanish]; 4H-Pyrido[1,2-a]pyrimidin-4-one,3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-2,9-dimethyl-; Ocaperidone (USAN); Ocaperidone [USAN:INN:BAN]; 3-[2-[4-(6-Fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl]ethyl]-2,9-dimethyl-4H-pyrido[1,2-a]pyrimidin-4-one; Ocaperidona; 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2,9-dimethylpyrido[1,2-a]pyrimidin-4-one; 8-[2-[4-(6-fluorobenzo[d]isoxazol-3-yl)-1-piperidyl]ethyl]-2,9-dimethyl-6,10-diazabicyclo[440]deca-2,4,8,10-tetraen-7-one; FG-3019
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Idiopathic pulmonary fibrosis [ICD-11: CB03.4; ICD-10: J84.1] | Phase 2 | [1] | |
| Pancreatic cancer [ICD-11: 2C10] | Phase 2 | [2] | ||
| Schizoaffective disorder [ICD-11: 6A21] | Phase 2 | [3], [4], [5] | ||
| Schizophrenia [ICD-11: 6A20] | Phase 2 | [3], [4], [5] | ||
| Type-2 diabetes [ICD-11: 5A11] | Phase 2 | [1] | ||
| Diabetic nephropathy [ICD-11: GB61.Z; ICD-9: 250.4] | Phase 1b | [1] | ||
| Company |
Johnson & Johnson
|
|||
| Structure |
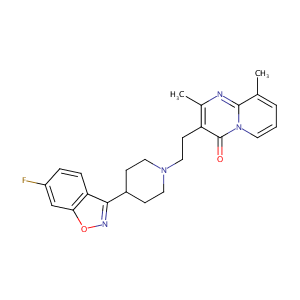 |
Download2D MOL |
||
| Formula |
C24H25FN4O2
|
|||
| Canonical SMILES |
CC1=CC=CN2C1=NC(=C(C2=O)CCN3CCC(CC3)C4=NOC5=C4C=CC(=C5)F)C
|
|||
| InChI |
1S/C24H25FN4O2/c1-15-4-3-10-29-23(15)26-16(2)19(24(29)30)9-13-28-11-7-17(8-12-28)22-20-6-5-18(25)14-21(20)31-27-22/h3-6,10,14,17H,7-9,11-13H2,1-2H3
|
|||
| InChIKey |
ZZQNEJILGNNOEP-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 129029-23-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Agents in development for the treatment of diabetic nephropathy. Expert Opin Emerg Drugs. 2008 Sep;13(3):447-63. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 46). | |||
| REF 4 | The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007 Oct;12(10):904-22. | |||
| REF 5 | Pharmacological profile of the new potent neuroleptic ocaperidone (R 79,598). J Pharmacol Exp Ther. 1992 Jan;260(1):146-59. | |||
| REF 6 | Current and emerging drugs for idiopathic pulmonary fibrosis. Expert Opin Emerg Drugs. 2007 Nov;12(4):627-46. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

