Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0SB6Y
|
||||
| Former ID |
DNC013560
|
||||
| Drug Name |
D-211A
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [529287] | ||
| Structure |
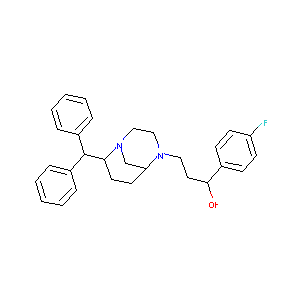
|
Download2D MOL |
|||
| Formula |
C29H33FN2O
|
||||
| Canonical SMILES |
C1CC(N2CCN(C1C2)CCC(C3=CC=C(C=C3)F)O)C(C4=CC=CC=C4)C5=C<br />C=CC=C5
|
||||
| InChI |
1S/C29H33FN2O/c30-25-13-11-22(12-14-25)28(33)17-18-31-19-20-32-21-26(31)15-16-27(32)29(23-7-3-1-4-8-23)24-9-5-2-6-10-24/h1-14,26-29,33H,15-21H2/t26-,27-,28+/m0/s1
|
||||
| InChIKey |
GAKAYJNKCDLKCE-HZFUHODCSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Sodium-dependent serotonin transporter | Target Info | Inhibitor | [529287] | |
| Sodium-dependent dopamine transporter | Target Info | Inhibitor | [529287] | ||
| Sodium-dependent noradrenaline transporter | Target Info | Inhibitor | [529287] | ||
| NetPath Pathway | TCR Signaling Pathway | ||||
| PANTHER Pathway | 5HT1 type receptor mediated signaling pathway | ||||
| 5HT2 type receptor mediated signaling pathway | |||||
| 5HT3 type receptor mediated signaling pathway | |||||
| 5HT4 type receptor mediated signaling pathwayP00001:Adrenaline and noradrenaline biosynthesis | |||||
| Parkinson disease | |||||
| Dopamine receptor mediated signaling pathwayP00001:Adrenaline and noradrenaline biosynthesis | |||||
| Pathway Interaction Database | Alpha-synuclein signaling | ||||
| WikiPathways | Monoamine Transport | ||||
| SIDS Susceptibility Pathways | |||||
| NRF2 pathway | |||||
| Synaptic Vesicle Pathway | |||||
| Serotonin Transporter ActivityWP727:Monoamine Transport | |||||
| Dopaminergic Neurogenesis | |||||
| Parkinsons Disease Pathway | |||||
| Transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compounds | |||||
| Neurotransmitter Clearance In The Synaptic CleftWP727:Monoamine Transport | |||||
| References | |||||
| Ref 529287 | Bioorg Med Chem. 2008 Mar 15;16(6):2769-78. Epub 2008 Jan 11.Further structural optimization of cis-(6-benzhydryl-piperidin-3-yl)-benzylamine and 1,4-diazabicyclo[3.3.1]nonane derivatives by introducing an exocyclic hydroxyl group: interaction with dopamine, serotonin, and norepinephrine transporters. | ||||
| Ref 529287 | Bioorg Med Chem. 2008 Mar 15;16(6):2769-78. Epub 2008 Jan 11.Further structural optimization of cis-(6-benzhydryl-piperidin-3-yl)-benzylamine and 1,4-diazabicyclo[3.3.1]nonane derivatives by introducing an exocyclic hydroxyl group: interaction with dopamine, serotonin, and norepinephrine transporters. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

