| Drug General Information |
| Drug ID |
D0A2FO
|
| Former ID |
DNC003744
|
| Drug Name |
(+/-)-7-methoxy-2-phenylchroman-4-one
|
| Synonyms |
7-methoxy-2-phenylchroman-4-one
|
| Indication |
Discovery agent
|
Investigative |
[1]
|
|---|
| Structure |
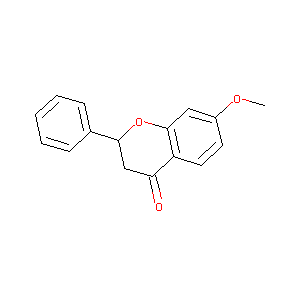
|
Download
2D MOL
3D MOL
|
| Canonical SMILES |
COC1=CC2=C(C=C1)C(=O)CC(O2)C3=CC=CC=C3
|
| InChI |
1S/C16H14O3/c1-18-12-7-8-13-14(17)10-15(19-16(13)9-12)11-5-3-2-4-6-11/h2-9,15H,10H2,1H3
|
| InChIKey |
VYESEQLQFXUROZ-UHFFFAOYSA-N
|
| Target and Pathway |
| Target(s) |
Cytochrome P450 19 |
Target Info |
Inhibitor |
[2]
|
|---|
| Amine oxidase [flavin-containing] B |
Target Info |
Inhibitor |
[1]
|
|
BioCyc Pathway
|
Superpathway of steroid hormone biosynthesis
|
|
Estradiol biosynthesis II
|
|
Estradiol biosynthesis IPWY66-401:Superpathway of tryptophan utilization
|
|
Tryptophan degradation via tryptamine
|
|
Dopamine degradation
|
|
Putrescine degradation III
|
|
Noradrenaline and adrenaline degradation
|
|
KEGG Pathway
|
Steroid hormone biosynthesis
|
|
Metabolic pathways
|
|
Ovarian steroidogenesishsa00260:Glycine, serine and threonine metabolism
|
|
Arginine and proline metabolism
|
|
Histidine metabolism
|
|
Tyrosine metabolism
|
|
Phenylalanine metabolism
|
|
Tryptophan metabolism
|
|
Drug metabolism - cytochrome P450
|
|
Serotonergic synapse
|
|
Dopaminergic synapse
|
|
Cocaine addiction
|
|
Amphetamine addiction
|
|
Alcoholism
|
|
NetPath Pathway
|
FSH Signaling Pathway
|
|
PANTHER Pathway
|
Androgen/estrogene/progesterone biosynthesisP00001:Adrenaline and noradrenaline biosynthesis
|
|
5-Hydroxytryptamine degredation
|
|
Dopamine receptor mediated signaling pathway
|
|
Pathway Interaction Database
|
Alpha-synuclein signaling
|
|
PathWhiz Pathway
|
Androgen and Estrogen Metabolism
|
|
Reactome
|
Endogenous sterols
|
|
WikiPathways
|
Metapathway biotransformation
|
|
Tryptophan metabolism
|
|
Oxidation by Cytochrome P450
|
|
Ovarian Infertility Genes
|
|
Metabolism of steroid hormones and vitamin D
|
|
FSH signaling pathway
|
|
Integrated Breast Cancer Pathway
|
|
Phase 1 - Functionalization of compoundsWP465:Tryptophan metabolism
|
|
Dopamine metabolism
|
|
Phase 1 - Functionalization of compounds
|
| References |
| REF 1 | Bioorg Med Chem. 2010 Feb;18(3):1273-9. Epub 2010 Jan 4.A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. |
|---|
| REF 2 | Bioorg Med Chem. 2008 Feb 1;16(3):1474-80. Epub 2007 Oct 22.New 7,8-benzoflavanones as potent aromatase inhibitors: synthesis and biological evaluation. |
|---|