Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0C5XC
|
||||
| Former ID |
DAP000322
|
||||
| Drug Name |
Clemastine
|
||||
| Synonyms |
Clemastina; Clemastinum; Meclastin; Meclastine; Mecloprodin; Tavegyl; Tavist; CLEMASTINE FUMARATE; HS 592; Clemastina [INN-Spanish]; Clemastine [USAN:BAN]; Clemastinum [INN-Latin]; HS-592; Tavegyl (TN); Tavist (TN); Tavist (*Fumarate*); (+)-(2R)-2-(2-(((R)-p-Chloro-alpha-methyl-alpha-phenylbenzyl)oxy)ethyl)-1-methylpyrrolidine; (+)-(2R)-2-[2-[[(R)-p-Chloro-alpha-methyl-alpha-phenylbenzyl]oxy]ethyl]-1-methylpyrrolidine; (2R)-2-(2-{[(1R)-1-(4-chlorophenyl)-1-phenylethyl]oxy}ethyl)-1-methylpyrrolidine; (2R)-2-[2-[(1R)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methylpyrrolidine; (2R)-2-{2-[(1R)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine; 2(R)-[2-[(1R)-(4-Chlorophenyl)-1-phenyl-ethoxy]ethyl-1-methylpyrrolidine; 2-(2-(4-Chlor-alpha-methylbenzhydryloxy)ethyl)-1-methylpyrrolidin
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Allergic rhinitis [ICD9: 472.0, 477, 995.3; ICD10:J00, J30, J31.0, T78.4] | Approved | [1], [2] | ||
| Therapeutic Class |
Antiallergic Agents
|
||||
| Company |
Norvatis Phamaceuticals Corporation
|
||||
| Structure |
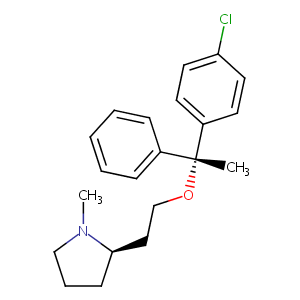
|
Download2D MOL |
|||
| Formula |
C21H26ClNO
|
||||
| InChI |
InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1
|
||||
| InChIKey |
YNNUSGIPVFPVBX-NHCUHLMSSA-N
|
||||
| CAS Number |
CAS 15686-51-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
597386, 7978959, 8169501, 11111002, 11112618, 11114089, 11335460, 11360699, 11371365, 11374708, 11461671, 11466334, 11467454, 11484553, 11486227, 11488505, 11490169, 11492770, 14875878, 34669560, 46506492, 47216681, 47440147, 48035000, 48035001, 48035002, 48334381, 48334382, 48415792, 49698428, 50038481, 50297783, 85788464, 92308858, 92729734, 93166549, 96079549, 103605085, 104105756, 104298787, 117462440, 124882478, 135021180, 136018285, 137001887, 141654852, 152146162, 160963631, 162180590, 162224684
|
||||
| SuperDrug ATC ID |
D04AA14; R06AA04
|
||||
| SuperDrug CAS ID |
cas=015686518
|
||||
| Target and Pathway | |||||
| Target(s) | Histamine H1 receptor | Target Info | Antagonist | [3], [4] | |
| KEGG Pathway | Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Inflammatory mediator regulation of TRP channels | |||||
| PANTHER Pathway | Histamine H1 receptor mediated signaling pathway | ||||
| Reactome | Histamine receptors | ||||
| G alpha (q) signalling events | |||||
| WikiPathways | Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | |||||
| IL-4 Signaling Pathway | |||||
| Gastrin-CREB signalling pathway via PKC and MAPK | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| References | |||||
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 073283. | ||||
| REF 2 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6063). | ||||
| REF 3 | Stereoselective synthesis of (-)-hydroxyclemastine as a versatile intermediate for the H1 receptor antagonist clemastine. Arch Pharm Res. 2007 Dec;30(12):1521-5. | ||||
| REF 4 | Histamine upregulates keratinocyte MMP-9 production via the histamine H1 receptor. J Invest Dermatol. 2008 Dec;128(12):2783-91. Epub 2008 Jun 12. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

