Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0C1WH
|
||||
| Former ID |
DAP000626
|
||||
| Drug Name |
Letrozole
|
||||
| Synonyms |
Femara; Femera; Letoval; Letrozol; Novartis Brand of Letrozole; CGS 20267; CGS-20267; FEM-345; Femara (TN); Letrozole [USAN:INN]; CGS 20267, Femara, Piroxicam, Letrozole; Letrozole (JAN/USP/INN); 1-[Bis-(4-cyanophenyl)methyl]-1,2,4-triazole; 1-[bis(4-cyanophenyl)methyl]-1,2,4-triazole; 4,4'-((1h-1,2,4-triazol-1-yl)methylene)dibenzonitrile; 4,4'-(1H-1,2,4-Triazol-1-ylmethylene)dibenzonitrile; 4,4'-(1H-1,2,4-triazol-1-yl-methylene)-bis(benzonitrile); 4,4'-(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitrile; 4,4'-(1H-1,2,4-triazol-1-ylmethylene)bis-Benzonitrile Letrozole; 4,4'-(1h-1,2,4-triazol-1-ylmethylene) bis-benzonitrile; 4,4'-(1h-1,2,4-triazol-1-ylmethylene)bis-benzonitrile; 4,4'-(1h-1,2,4-triazol-1-ylmethylene)bisbenzonitrile; 4-[(4-cyanophenyl)-(1,2,4-triazol-1-yl)methyl]benzonitrile
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Hormonally-responsive breast cancer [ICD9: 174, 175; ICD10:C50] | Approved | [1], [2] | ||
| Therapeutic Class |
Anticancer Agents
|
||||
| Structure |
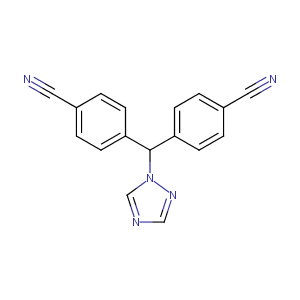
|
Download2D MOL |
|||
| Formula |
C17H11N5
|
||||
| InChI |
InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H
|
||||
| InChIKey |
HPJKCIUCZWXJDR-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 112809-51-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10363, 535027, 6899006, 7848027, 7978496, 8152457, 11112889, 11467053, 11468173, 11486806, 12014531, 14799856, 26719850, 29223016, 46386665, 46504610, 46513479, 47440364, 47736586, 47810851, 47959864, 48416161, 49681588, 49699256, 49830389, 49888730, 50100535, 53789196, 56311274, 56312145, 56313556, 56313557, 56313754, 56352886, 57322043, 71821268, 79643905, 81040865, 81092820, 85787542, 92126055, 92308318, 92308918, 92712289, 93166173, 93167148, 99355691, 99437044, 103513368, 103914616
|
||||
| ChEBI ID |
ChEBI:6413
|
||||
| SuperDrug ATC ID |
L02BG04
|
||||
| SuperDrug CAS ID |
cas=112809515
|
||||
| Target and Pathway | |||||
| Target(s) | Cytochrome P450 19 | Target Info | Inhibitor | [3] | |
| BioCyc Pathway | Superpathway of steroid hormone biosynthesis | ||||
| Estradiol biosynthesis II | |||||
| Estradiol biosynthesis I | |||||
| KEGG Pathway | Steroid hormone biosynthesis | ||||
| Metabolic pathways | |||||
| Ovarian steroidogenesis | |||||
| NetPath Pathway | FSH Signaling Pathway | ||||
| PANTHER Pathway | Androgen/estrogene/progesterone biosynthesis | ||||
| PathWhiz Pathway | Androgen and Estrogen Metabolism | ||||
| Reactome | Endogenous sterols | ||||
| WikiPathways | Metapathway biotransformation | ||||
| Tryptophan metabolism | |||||
| Oxidation by Cytochrome P450 | |||||
| Ovarian Infertility Genes | |||||
| Metabolism of steroid hormones and vitamin D | |||||
| FSH signaling pathway | |||||
| Integrated Breast Cancer Pathway | |||||
| Phase 1 - Functionalization of compounds | |||||
| References | |||||
| REF 1 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5209). | ||||
| REF 2 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | ||||
| REF 3 | Aromatase inhibitors--theoretical concept and present experiences in the treatment of endometriosis. Zentralbl Gynakol. 2003 Jul-Aug;125(7-8):247-51. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

