| Drug General Information |
| Drug ID |
D09QDY
|
| Former ID |
DNC004644
|
| Drug Name |
5-Indan-(1Z)-ylidenemethyl-1H-imidazole
|
| Drug Type |
Small molecular drug
|
| Indication |
Discovery agent
|
Investigative |
[1]
|
|---|
| Structure |
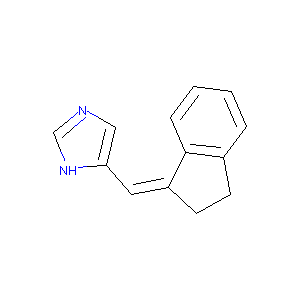
|
Download
2D MOL
3D MOL
|
| Formula |
C13H12N2
|
| Canonical SMILES |
C1CC(=CC2=CN=CN2)C3=CC=CC=C31
|
| InChI |
1S/C13H12N2/c1-2-4-13-10(3-1)5-6-11(13)7-12-8-14-9-15-12/h1-4,7-9H,5-6H2,(H,14,15)/b11-7-
|
| InChIKey |
FXLOWXDBTSZJFZ-XFFZJAGNSA-N
|
| PubChem Compound ID |
|
| Target and Pathway |
| Target(s) |
Cytochrome P450 11B1, mitochondrial |
Target Info |
Inhibitor |
[1]
|
|---|
| Cytochrome P450 19 |
Target Info |
Inhibitor |
[1]
|
|
BioCyc Pathway
|
Superpathway of steroid hormone biosynthesis
|
|
Glucocorticoid biosynthesis
|
|
Mineralocorticoid biosynthesisPWY-7305:Superpathway of steroid hormone biosynthesis
|
|
Estradiol biosynthesis II
|
|
Estradiol biosynthesis I
|
|
KEGG Pathway
|
Steroid hormone biosynthesis
|
|
Metabolic pathwayshsa00140:Steroid hormone biosynthesis
|
|
Metabolic pathways
|
|
Ovarian steroidogenesis
|
|
NetPath Pathway
|
FSH Signaling Pathway
|
|
PANTHER Pathway
|
Androgen/estrogene/progesterone biosynthesis
|
|
PathWhiz Pathway
|
SteroidogenesisPW000045:Androgen and Estrogen Metabolism
|
|
Reactome
|
Glucocorticoid biosynthesis
|
|
Endogenous sterolsR-HSA-211976:Endogenous sterols
|
|
WikiPathways
|
Metapathway biotransformation
|
|
Oxidation by Cytochrome P450
|
|
Metabolism of steroid hormones and vitamin D
|
|
Corticotropin-releasing hormoneWP702:Metapathway biotransformation
|
|
Tryptophan metabolism
|
|
Ovarian Infertility Genes
|
|
FSH signaling pathway
|
|
Integrated Breast Cancer Pathway
|
|
Phase 1 - Functionalization of compounds
|
| References |
| REF 1 | J Med Chem. 2005 Mar 24;48(6):1796-805.Synthesis and evaluation of imidazolylmethylenetetrahydronaphthalenes and imidazolylmethyleneindanes: potent inhibitors of aldosterone synthase. |
|---|