Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05VLS
|
||||
| Former ID |
DAP001338
|
||||
| Drug Name |
Rofecoxib
|
||||
| Synonyms |
Ceoxx; Refecoxib; Vioxx; Cahill May Roberts brand of rofecoxib; MSD brand of rofecoxib; Merck Frosst brandof rofecoxib; Merck brand of rofecoxib; Vioxx Dolor; MK 0966; MK 0996; MK 966; MK 996; MK0966; Ceeoxx (TN); Ceoxx (TN); KS-1107; MK-0966; MK-966; Merck Sharp & Dhome brand of rofecoxib; Vioxx (TN); Vioxx (trademark); Rofecoxib (JAN/USAN/INN); 3-(4-methylsulfonylphenyl)-4-phenyl-2H-furan-5-one; 3-Phenyl-4-(4-(methylsulfonyl)phenyl))-2(5H)-furanone; 3-phenyl-4-[4-(methylsulfonyl)phenyl]-2(5H)-furanone; 4-(4-(Methylsulfonyl)phenyl)-3-phenyl-2(5H)-furanone; 4-(4-methylsulfonylphenyl)-3-phenyl-5H-furan-2-one; 4-(p-(Methylsulfonyl)phenyl)-3-phenyl-2(5H)-furanone; 4-[4-(methylsulfonyl)phenyl]-3-phenyl-2(5H)-furanone; 4-[4-(methylsulfonyl)phenyl]-3-phenylfuran-2(5H)-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiinflammatory Agents
|
||||
| Company |
Merck & Co
|
||||
| Structure |
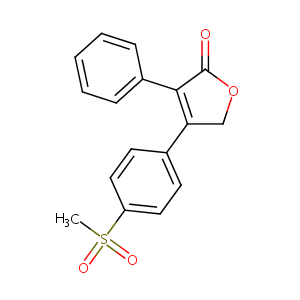
|
Download2D MOL |
|||
| Formula |
C17H14O4S
|
||||
| InChI |
InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3
|
||||
| InChIKey |
RZJQGNCSTQAWON-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 162011-90-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9792, 535364, 5146347, 7847634, 7980536, 8146414, 8153131, 11341940, 11362123, 11362973, 11364757, 11365535, 11367319, 11368097, 11369881, 11372304, 11372922, 11374381, 11375481, 11376259, 11378046, 11445527, 11484228, 11487525, 11488262, 11490968, 11492446, 11493933, 11528653, 12014954, 14752370, 25819946, 26612684, 26680170, 26719838, 26748942, 26748943, 29224158, 46386634, 46504787, 47365310, 47810857, 48334605, 48416526, 49665949, 49666006, 49681737, 49846184, 50107491, 50107492
|
||||
| ChEBI ID |
ChEBI:8887
|
||||
| SuperDrug ATC ID |
M01AH02
|
||||
| SuperDrug CAS ID |
cas=162011907
|
||||
| Target and Pathway | |||||
| KEGG Pathway | Arachidonic acid metabolism | ||||
| Metabolic pathways | |||||
| NF-kappa B signaling pathway | |||||
| VEGF signaling pathway | |||||
| TNF signaling pathway | |||||
| Retrograde endocannabinoid signaling | |||||
| Serotonergic synapse | |||||
| Ovarian steroidogenesis | |||||
| Oxytocin signaling pathway | |||||
| Regulation of lipolysis in adipocytes | |||||
| Leishmaniasis | |||||
| Pathways in cancer | |||||
| Chemical carcinogenesis | |||||
| MicroRNAs in cancer | |||||
| Small cell lung cancer | |||||
| PathWhiz Pathway | Arachidonic Acid Metabolism | ||||
| References | |||||
| Ref 536772 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | ||||
| Ref 539916 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2893). | ||||
| Ref 535528 | Clinical experience with cyclooxygenase-2 inhibitors. Rheumatology (Oxford). 2002 Apr;41 Supp 1:16-22; discussion 35-42. | ||||
| Ref 535660 | Knockouts model the 100 best-selling drugs--will they model the next 100? Nat Rev Drug Discov. 2003 Jan;2(1):38-51. | ||||
| Ref 535785 | The effect of the selective cyclooxygenase-2 inhibitor rofecoxib on human colorectal cancer liver metastases. Gastroenterology. 2003 Sep;125(3):716-29. | ||||
| Ref 535836 | Aromatase inhibitors--theoretical concept and present experiences in the treatment of endometriosis. Zentralbl Gynakol. 2003 Jul-Aug;125(7-8):247-51. | ||||
| Ref 536549 | Privileged structures: a useful concept for the rational design of new lead drug candidates. Mini Rev Med Chem. 2007 Nov;7(11):1108-19. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

