| Drug General Information |
| Drug ID |
D03SAV
|
| Former ID |
DNC004906
|
| Drug Name |
ICI-199441
|
| Drug Type |
Small molecular drug
|
| Indication |
Discovery agent
|
Investigative |
[1]
|
|---|
| Structure |
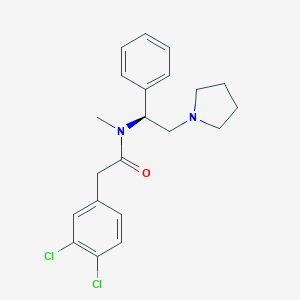
|
Download
2D MOL
3D MOL
|
| Formula |
C21H25Cl3N2O
|
| Canonical SMILES |
CN(C(CN1CCCC1)C2=CC=CC=C2)C(=O)CC3=CC(=C(C=C3)Cl)Cl
|
| InChI |
1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3
|
| InChIKey |
AEJOEPSMZCEYJN-UHFFFAOYSA-N
|
| PubChem Compound ID |
|
| Target and Pathway |
| Target(s) |
Urotensin II receptor |
Target Info |
Inhibitor |
[2]
|
|---|
| Delta-type opioid receptor |
Target Info |
Inhibitor |
[3]
|
| Kappa-type opioid receptor |
Target Info |
Inhibitor |
[1]
|
| Cytochrome P450 2D6 |
Target Info |
Inhibitor |
[3]
|
| Mu-type opioid receptor |
Target Info |
Inhibitor |
[4]
|
|
KEGG Pathway
|
Neuroactive ligand-receptor interactionhsa04022:cGMP-PKG signaling pathway
|
|
Sphingolipid signaling pathway
|
|
Neuroactive ligand-receptor interactionhsa04080:Neuroactive ligand-receptor interactionhsa00980:Metabolism of xenobiotics by cytochrome P450
|
|
Drug metabolism - cytochrome P450
|
|
Serotonergic synapsehsa04080:Neuroactive ligand-receptor interaction
|
|
Estrogen signaling pathway
|
|
Morphine addiction
|
|
NetPath Pathway
|
TCR Signaling Pathway
|
|
PANTHER Pathway
|
Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway
|
|
Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway
|
|
Enkephalin release
|
|
Opioid proenkephalin pathway
|
|
Opioid proopiomelanocortin pathwayP00026:Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway
|
|
Opioid prodynorphin pathwayP00026:Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway
|
|
Pathway Interaction Database
|
IL4-mediated signaling events
|
|
Reactome
|
Peptide ligand-binding receptors
|
|
G alpha (q) signalling eventsR-HSA-375276:Peptide ligand-binding receptors
|
|
G alpha (i) signalling eventsR-HSA-375276:Peptide ligand-binding receptors
|
|
G alpha (i) signalling eventsR-HSA-211981:XenobioticsR-HSA-375276:Peptide ligand-binding receptors
|
|
G alpha (i) signalling events
|
|
WikiPathways
|
Gastrin-CREB signalling pathway via PKC and MAPK
|
|
GPCR ligand binding
|
|
GPCR downstream signaling
|
|
GPCRs, OtherWP455:GPCRs, Class A Rhodopsin-like
|
|
Peptide GPCRs
|
|
GPCR downstream signalingWP455:GPCRs, Class A Rhodopsin-like
|
|
GPCR downstream signalingWP702:Metapathway biotransformation
|
|
Tamoxifen metabolism
|
|
Oxidation by Cytochrome P450
|
|
Vitamin D Receptor Pathway
|
|
Aripiprazole Metabolic Pathway
|
|
Fatty Acid Omega Oxidation
|
|
Codeine and Morphine MetabolismWP69:TCR Signaling Pathway
|
|
GPCRs, Class A Rhodopsin-like
|
|
Opioid Signalling
|
| References |
| REF 1 | J Med Chem. 1996 Apr 12;39(8):1729-35.Arylacetamide-derived fluorescent probes: synthesis, biological evaluation, and direct fluorescent labeling of kappa opioid receptors in mouse microglial cells. |
|---|
| REF 2 | Bioorg Med Chem Lett. 2008 Jul 1;18(13):3716-9. Epub 2008 May 20.Potent and selective small-molecule human urotensin-II antagonists with improved pharmacokinetic profiles. |
|---|
| REF 3 | Bioorg Med Chem Lett. 2005 May 16;15(10):2647-52.Arylacetamide kappa opioid receptor agonists with reduced cytochrome P450 2D6 inhibitory activity. |
|---|
| REF 4 | J Med Chem. 1994 Sep 2;37(18):2856-64.Isothiocyanate-substituted kappa-selective opioid receptor ligands derived from N-methyl-N-[(1S)-1-phenyl-2-(1-pyrrolidinyl)ethyl] phenylacetamide. |
|---|