Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0YA2L
|
||||
| Former ID |
DAP000396
|
||||
| Drug Name |
Teriparatide
|
||||
| Synonyms |
Forteo; Forteo (TN); Teriparatide (genetical recombination); Teriparatide (USAN/INN); Teriparatide (genetical recombination) (JAN)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Bone Density Conservation Agents
|
||||
| Company |
Eli Lilly
|
||||
| Structure |
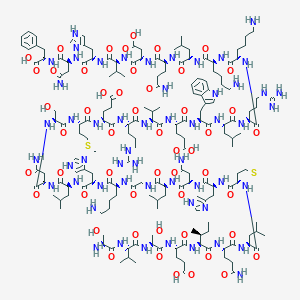
|
Download2D MOL |
|||
| Formula |
C181H291N55O51S2
|
||||
| CAS Number |
CAS 52232-67-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
H05AA02
|
||||
| SuperDrug CAS ID |
cas=052232674
|
||||
| Target and Pathway | |||||
| Target(s) | Parathyroid hormone ligand | Target Info | Modulator | [556264] | |
| References | |||||
| Ref 467716 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4448). | ||||
| Ref 536361 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. Epub 2007 Feb 20. | ||||
| Ref 1572605 | The ChEMBL database in 2017. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

