Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04BNP
|
||||
| Former ID |
DAP000238
|
||||
| Drug Name |
Temazepam
|
||||
| Synonyms |
Cerepax; Crisonar; Dasuen; Euhypnos; Euipnos; Gelthix; Hydroxydiazepam; Lenal; Levanxene; Levanxol; Levanzene; Mabertin; Methyloxazepam; Nocturne; Nomapam; Normison; Normitab; Nortem; Oxydiazepam; Perdorm; Planum; Remestan; Restoril; Signopam; Temador; Temaz; Temaze; Temazepamum; Temtabs; Tenox; AHP Brand of Temazepam; Alphapharm Brand of Temazepam; Apo Temazepam; Apotex Brand of Temazepam; Ct Arzneimittel Brand of Temazepam; Desitin Brand of Temazepam; Gen Temazepam; Genopharm Brand of Temazepam; Genpharm Brand of Temazepam; ICN Brand of Temazepam; Katwijk Brand of Temazepam; Knoll Brand of Temazepam; Mallinckrodt Brand of Temazepam; Neodorm SP; Norkotral Tema; Norton Brand of Temazepam; Novartis Brand of Temazepam; Novo Temazepam; Novopharm Brand of Temazepam; Nu Pharm Brand of Temazepam; Nu Temazepam; Orion Brand of Temazepam; PMS Temazepam; Pharmascience Brand of Temazepam; Pronervon T; Scheffler Brand of Temazepam; Sigma Brand of Temazepam; Temazep von ct; Uvamin Retard; Wyeth Brand of Temazepam; ER 115; K3917; Pfizer Brand 1 of Temazepam; Pfizer Brand 2 of Temazepam; Ro 5 5345; Ro55345; SaH 47 603; SaH 47603; WY 2917; WY 3917; WY3917; Apo-Temazepam; Ct-Arzneimittel Brand of Temazepam; Euhypnos (TN); Gen-Temazepam; K-3917; N-Methyloxazepam; Norkotral (TN); Normison (TN); Novo-Temazepam; Nu-Pharm Brand of Temazepam; Nu-Temazepam; PMS-Temazepam; Remestan (TN); Restoril (TN); Ro 5-5345; SaH 47-603; Tema, Norkotral; Temazepam, pharmaceutical grade; Temazepamum [INN-Latin]; Temtabs (TN); Tenox (TN); Wy-3917; Ro-5-5345; Temazepam (USP/INN); Temazepam [USAN:INN:BAN]; 1,3-Dihydro-7-chloro-3-hydroxy-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one; 3 Hydroxydiazepam; 3-Hydroxydiazepam; 7-CHLORO-1,3-DIHYDRO-3-HYDROXY-1-METHYL-5-PHENYL-2H-1,4-BENZODIAZEPIN-2-ONE; 7-Chloro-3-hydroxy-1-methyl-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one; 7-chloro-3-hydroxy-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||
| Company |
Mylan Inc
|
||||
| Structure |
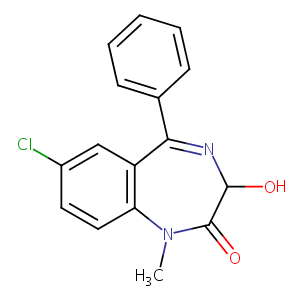
|
Download2D MOL |
|||
| Formula |
C16H13ClN2O2
|
||||
| InChI |
InChI=1S/C16H13ClN2O2/c1-19-13-8-7-11(17)9-12(13)14(18-15(20)16(19)21)10-5-3-2-4-6-10/h2-9,15,20H,1H3
|
||||
| InChIKey |
SEQDDYPDSLOBDC-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 846-50-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9335, 136538, 3249168, 4624912, 7847436, 7980739, 8150144, 8153314, 10536883, 11336164, 11361403, 11462375, 14873813, 24900518, 29215382, 29224443, 46506604, 47216853, 48414499, 48416596, 49857686, 50335785, 57322751, 57654708, 92729793, 99302077, 103252229, 104309107, 124892195, 126674763, 129225114, 134337552, 134979721, 136949648, 137128143, 143325958, 144205223, 144207242, 160963579, 163357056, 164765243, 175611282, 176262034, 178103873, 179116909, 198991797, 223441048, 223682944, 224807633, 226416533
|
||||
| SuperDrug ATC ID |
N05CD07
|
||||
| SuperDrug CAS ID |
cas=000846504
|
||||
| Target and Pathway | |||||
| Target(s) | Peripheral-type benzodiazepine receptor | Target Info | Modulator | [556264] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| HTLV-I infection | |||||
| References | |||||
| Ref 538229 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 070919. | ||||
| Ref 542322 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7300). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

