Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0O7DA
|
||||
| Former ID |
DNC003916
|
||||
| Drug Name |
(+)-BUTACLAMOL
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
| Structure |
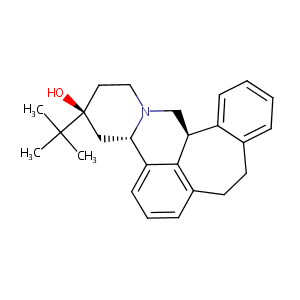
|
Download2D MOL |
|||
| Formula |
C25H31NO
|
||||
| InChI |
InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1
|
||||
| InChIKey |
ZZJYIKPMDIWRSN-HWBMXIPRSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | D(3) dopamine receptor | Target Info | Inhibitor | [2] | |
| D(2) dopamine receptor | Target Info | Inhibitor | [3] | ||
| Dopamine D1 receptor | Target Info | Inhibitor | [4] | ||
| Adenosine A3 receptor | Target Info | Inhibitor | [5] | ||
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| Dopaminergic synapsehsa04015:Rap1 signaling pathway | |||||
| cAMP signaling pathway | |||||
| Gap junction | |||||
| Dopaminergic synapse | |||||
| Parkinson's disease | |||||
| Cocaine addiction | |||||
| Alcoholismhsa04020:Calcium signaling pathway | |||||
| Amphetamine addiction | |||||
| Morphine addiction | |||||
| Alcoholism | |||||
| PANTHER Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | ||||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | |||||
| Dopamine receptor mediated signaling pathway | |||||
| Nicotine pharmacodynamics pathwayP05912:Dopamine receptor mediated signaling pathway | |||||
| PathWhiz Pathway | Dopamine Activation of Neurological Reward System | ||||
| Reactome | Dopamine receptors | ||||
| G alpha (i) signalling eventsR-HSA-390651:Dopamine receptors | |||||
| G alpha (s) signalling eventsR-HSA-417973:Adenosine P1 receptors | |||||
| G alpha (i) signalling events | |||||
| WikiPathways | Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| Nicotine Activity on Dopaminergic Neurons | |||||
| GPCRs, OtherWP666:Hypothetical Network for Drug Addiction | |||||
| Genes and (Common) Pathways Underlying Drug Addiction | |||||
| Nicotine Activity on Dopaminergic NeuronsWP666:Hypothetical Network for Drug Addiction | |||||
| GPCR downstream signalingWP80:Nucleotide GPCRs | |||||
| GPCRs, Other | |||||
| References | |||||
| REF 1 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 62). | ||||
| REF 2 | J Med Chem. 2008 Nov 27;51(22):7094-8.cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine (A-987306), a new histamine H4R antagonist that blocks pain responses against carrageenan-induced hyperalgesia. | ||||
| REF 3 | J Med Chem. 2005 Jan 13;48(1):266-73.Synthesis and structure-activity relationships of 1-aralkyl-4-benzylpiperidine and 1-aralkyl-4-benzylpiperazine derivatives as potent sigma ligands. | ||||
| REF 4 | J Med Chem. 2000 May 18;43(10):2079-81.7-Methyl-6,7,8,9,14,15-hexahydro-5H-benz[d]indolo[2,3-g]azecine: a new heterocyclic system and a new lead compound for dopamine receptor antagonists. | ||||
| REF 5 | J Med Chem. 2005 Nov 3;48(22):6887-96.2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and analogues as A2A adenosine receptor antagonists. Design, synthesis, and pharmacological characterization. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

