Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06QFO
|
||||
| Former ID |
DNC004861
|
||||
| Drug Name |
(5-(pyridin-3-yl)furan-2-yl)methanamine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
| Structure |
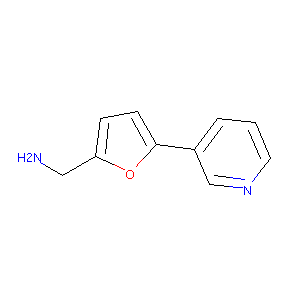
|
Download2D MOL |
|||
| Formula |
C10H10N2O
|
||||
| Canonical SMILES |
C1=CC(=CN=C1)C2=CC=C(O2)CN
|
||||
| InChI |
1S/C10H10N2O/c11-6-9-3-4-10(13-9)8-2-1-5-12-7-8/h1-5,7H,6,11H2
|
||||
| InChIKey |
LENAVORGWBTPJR-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Cytochrome P450 2D6 | Target Info | Inhibitor | [1] | |
| KEGG Pathway | Metabolism of xenobiotics by cytochrome P450 | ||||
| Drug metabolism - cytochrome P450 | |||||
| Serotonergic synapse | |||||
| Reactome | Xenobiotics | ||||
| WikiPathways | Metapathway biotransformation | ||||
| Tamoxifen metabolism | |||||
| Oxidation by Cytochrome P450 | |||||
| Vitamin D Receptor Pathway | |||||
| Aripiprazole Metabolic Pathway | |||||
| Fatty Acid Omega Oxidation | |||||
| Codeine and Morphine Metabolism | |||||
| References | |||||
| REF 1 | J Med Chem. 2006 Nov 30;49(24):6987-7001.Synthetic inhibitors of cytochrome P-450 2A6: inhibitory activity, difference spectra, mechanism of inhibition, and protein cocrystallization. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

