Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03QZB
|
||||
| Former ID |
DIB012733
|
||||
| Drug Name |
F-1394
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Arteriosclerosis [ICD9: 440; ICD10:I70] | Discontinued in Phase 1 | [1] | ||
| Company |
Fujirebio KK
|
||||
| Structure |
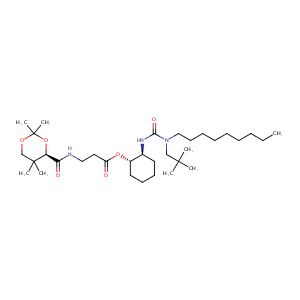
|
Download2D MOL |
|||
| Formula |
C33H61N3O6
|
||||
| Canonical SMILES |
C(=O)(N[C@@H]1[C@@H](OC(=O)CCNC(=O)[C@@H]2OC(OCC2(C)C)(<br />C)C)CCCC1)N(CC(C)(C)C)CCCCCCCCC
|
||||
| CAS Number |
CAS 162490-89-3
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Liver carboxylesterase | Target Info | Modulator | [2] | |
| KEGG Pathway | Drug metabolism - other enzymes | ||||
| Metabolic pathways | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| WikiPathways | NRF2 pathway | ||||
| Nuclear Receptors Meta-Pathway | |||||
| Heroin metabolism | |||||
| Irinotecan Pathway | |||||
| Fluoropyrimidine Activity | |||||
| Phase I biotransformations, non P450 | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003109) | ||||
| REF 2 | ACAT inhibitor F-1394 prevents intimal hyperplasia induced by balloon injury in rabbits. J Lipid Res. 2001 Apr;42(4):480-8. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

