Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T71377
(Former ID: TTDR00012)
|
|||||
| Target Name |
Interleukin 3 receptor alpha (IL3RA)
|
|||||
| Synonyms |
Interleukin-3 receptor subunit alpha; IL3R; IL-3RA; IL-3R-alpha; IL-3R subunit alpha; IL-3 receptor subunit alpha; CD123 antigen; CD123
Click to Show/Hide
|
|||||
| Gene Name |
IL3RA
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Acute myeloid leukaemia [ICD-11: 2A60] | |||||
| Function |
This is a receptor for interleukin-3.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MVLLWLTLLLIALPCLLQTKEDPNPPITNLRMKAKAQQLTWDLNRNVTDIECVKDADYSM
PAVNNSYCQFGAISLCEVTNYTVRVANPPFSTWILFPENSGKPWAGAENLTCWIHDVDFL SCSWAVGPGAPADVQYDLYLNVANRRQQYECLHYKTDAQGTRIGCRFDDISRLSSGSQSS HILVRGRSAAFGIPCTDKFVVFSQIEILTPPNMTAKCNKTHSFMHWKMRSHFNRKFRYEL QIQKRMQPVITEQVRDRTSFQLLNPGTYTVQIRARERVYEFLSAWSTPQRFECDQEEGAN TRAWRTSLLIALGTLLALVCVFVICRRYLVMQRLFPRIPHMKDPIGDSFQNDKLVVWEAG KAGLEECLVTEVQVVQKT Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Tagraxofusp | Drug Info | Approved | Blastic plasmacytoid dendritic cell neoplasm | [1] | |
| Clinical Trial Drug(s) | [+] 21 Clinical Trial Drugs | + | ||||
| 1 | CD123/CLL1 CAR-T Cells | Drug Info | Phase 2/3 | Acute myeloid leukaemia | [2] | |
| 2 | Flotetuzumab | Drug Info | Phase 2 | Acute myeloid leukaemia | [3] | |
| 3 | MB-102 | Drug Info | Phase 2 | Acute kidney injury | [4] | |
| 4 | Talacotuzumab | Drug Info | Phase 2 | Acute myeloid leukaemia | [5], [6] | |
| 5 | 4SCAR19 and 4SCAR123 | Drug Info | Phase 1/2 | B-cell lymphoma | [7] | |
| 6 | Anti-CD123-CAR-transduced T cells | Drug Info | Phase 1/2 | leukaemia | [8] | |
| 7 | CART-123 cells | Drug Info | Phase 1/2 | Acute myeloid leukaemia | [9] | |
| 8 | CD123-specific gene-engineered T cells | Drug Info | Phase 1/2 | Acute myeloid leukaemia | [10] | |
| 9 | SAR440234 | Drug Info | Phase 1/2 | Acute myeloid leukaemia | [11] | |
| 10 | Anti-CD123 CAR-T cells | Drug Info | Phase 1 | leukaemia | [12] | |
| 11 | APVO436 | Drug Info | Phase 1 | Acute myeloid leukaemia | [13] | |
| 12 | CART-123 cells | Drug Info | Phase 1 | Myelodysplastic syndrome | [14] | |
| 13 | CART123 cells | Drug Info | Phase 1 | Acute myeloid leukaemia | [15] | |
| 14 | CD123-CD33 Ccar | Drug Info | Phase 1 | Acute myeloid leukaemia | [16] | |
| 15 | CD123CAR-41BB-CD3zeta-EGFRt-expressing T cells | Drug Info | Phase 1 | Acute myeloid leukaemia | [17] | |
| 16 | CSL-362-AML | Drug Info | Phase 1 | leukaemia | [18] | |
| 17 | CSL311 | Drug Info | Phase 1 | Asthma | [19] | |
| 18 | IM23 | Drug Info | Phase 1 | Acute myeloid leukaemia | [20] | |
| 19 | JNJ-63709178 | Drug Info | Phase 1 | Acute myeloid leukaemia | [5], [21] | |
| 20 | MGD006 | Drug Info | Phase 1 | Acute myeloid lymphoma | [3], [22] | |
| 21 | UCART123 | Drug Info | Phase 1 | Acute myeloid leukaemia | [21] | |
| Preclinical Drug(s) | [+] 2 Preclinical Drugs | + | ||||
| 1 | Autologous Anti-CD 123 CAR TCR/4-1BB-expressing T-lymphocytes | Drug Info | Phase 0 | Acute myeloid leukaemia | [23] | |
| 2 | CAR-T cells targeting CD123 | Drug Info | Preclinical | Acute myeloid leukaemia | [24] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | Daniplestim | Drug Info | Terminated | Osteoporosis | [25] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Modulator | [+] 2 Modulator drugs | + | ||||
| 1 | Tagraxofusp | Drug Info | [1] | |||
| 2 | MGD006 | Drug Info | [22], [33] | |||
| CAR-T-Cell-Therapy(Dual specific) | [+] 2 CAR-T-Cell-Therapy(Dual specific) drugs | + | ||||
| 1 | CD123/CLL1 CAR-T Cells | Drug Info | [2] | |||
| 2 | 4SCAR19 and 4SCAR123 | Drug Info | [7] | |||
| CAR-T-Cell-Therapy | [+] 13 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | MB-102 | Drug Info | [5] | |||
| 2 | Anti-CD123-CAR-transduced T cells | Drug Info | [8] | |||
| 3 | CART-123 cells | Drug Info | [9] | |||
| 4 | CD123-specific gene-engineered T cells | Drug Info | [10] | |||
| 5 | Anti-CD123 CAR-T cells | Drug Info | [12] | |||
| 6 | Anti-CD123 CAR-T treatment | Drug Info | [27] | |||
| 7 | CART-123 cells | Drug Info | [14] | |||
| 8 | CART123 cells | Drug Info | [15] | |||
| 9 | CD123CAR-41BB-CD3zeta-EGFRt-expressing T cells | Drug Info | [17] | |||
| 10 | IM23 | Drug Info | [20] | |||
| 11 | UCART123 | Drug Info | [34], [35] | |||
| 12 | Autologous Anti-CD 123 CAR TCR/4-1BB-expressing T-lymphocytes | Drug Info | [23] | |||
| 13 | CAR-T cells targeting CD123 | Drug Info | [24] | |||
| Inhibitor | [+] 2 Inhibitor drugs | + | ||||
| 1 | APVO436 | Drug Info | [28] | |||
| 2 | JNJ-63709178 | Drug Info | [32] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | Daniplestim | Drug Info | [36] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Edetic acid | Ligand Info | |||||
| Structure Description | Cytokine-receptor complex | PDB:5UWC | ||||
| Method | X-ray diffraction | Resolution | 2.40 Å | Mutation | Yes | [37] |
| PDB Sequence |
ITNLRMKAKA
36 QQLTWECVKD55 ADYSMPAVNN65 SYCQFGAISL75 CEVTNYTVRV85 STWILFPENS 100 GKPWAGAENL110 TCWIHDVDFL120 SCSWAVGPGA130 PADVQYDLYL140 NVANRRQQYE 150 CLHYKTDAQG160 TRIGCRFDDI170 SRLSSGSQSS180 HILVRGRSAA190 FGIPCTDKFV 200 VFSQIEILTP210 PQMTAKCNKT220 HSFMHWKMRS230 HFNRKFRYEL240 QIQKRMQPVI 250 TEQVRDRTSF260 QLLNPGTYTV270 QIRARERVYE280 FLSAWSTPQR290 FEC |
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Interleukin 3 receptor (CSF2RB) | 23.554 (57/242) | 3.00E-03 | |
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Apoptosis | hsa04210 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
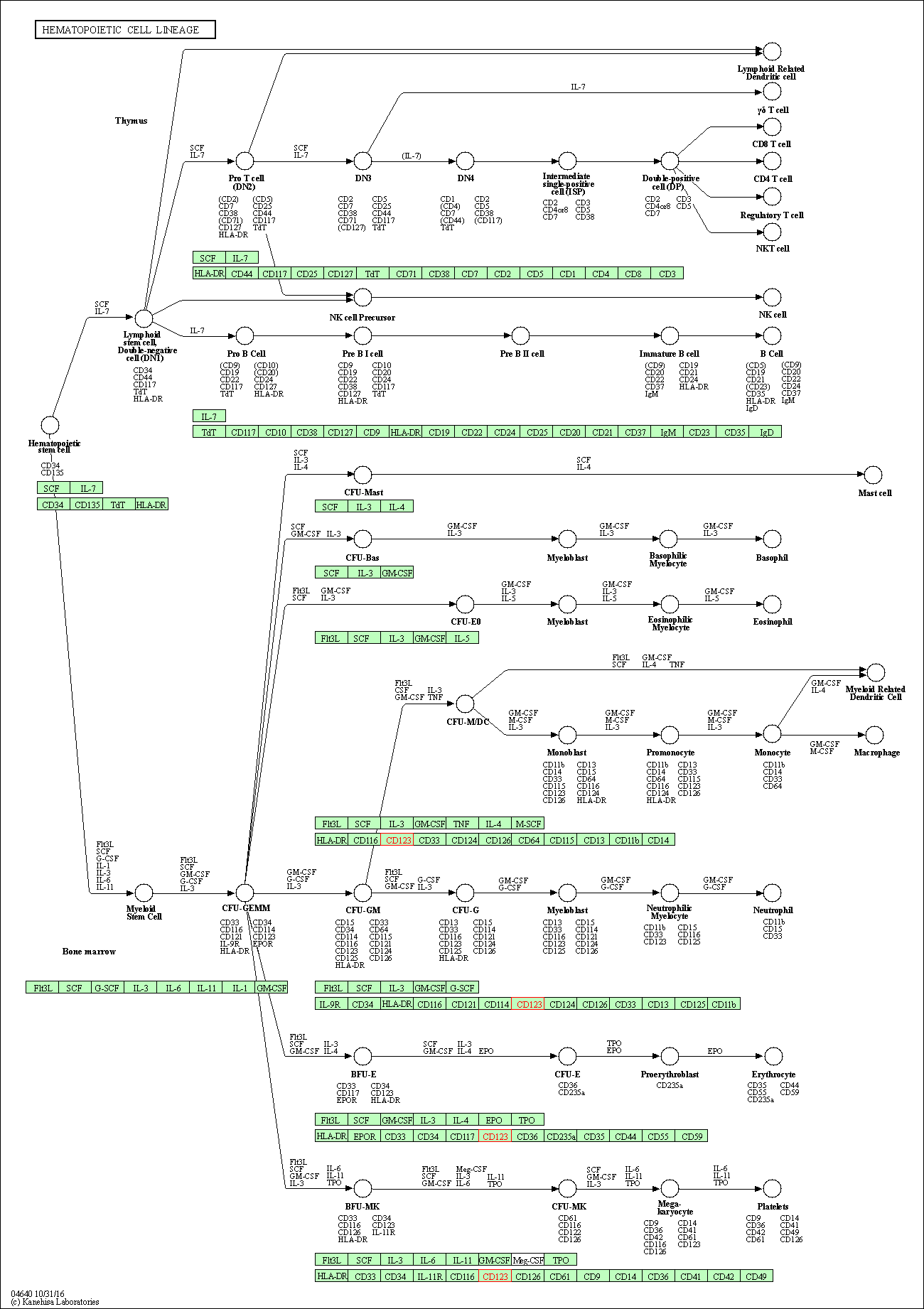
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 2 | Degree centrality | 2.15E-04 | Betweenness centrality | 0.00E+00 |
|---|---|---|---|---|---|
| Closeness centrality | 1.87E-01 | Radiality | 1.31E+01 | Clustering coefficient | 1.00E+00 |
| Neighborhood connectivity | 1.25E+01 | Topological coefficient | 6.58E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| 2 | PI3K-Akt signaling pathway | |||||
| 3 | Apoptosis | |||||
| 4 | Jak-STAT signaling pathway | |||||
| 5 | Hematopoietic cell lineage | |||||
| NetPath Pathway | [+] 2 NetPath Pathways | + | ||||
| 1 | IL5 Signaling Pathway | |||||
| 2 | IL3 Signaling Pathway | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | IL3-mediated signaling events | |||||
| Reactome | [+] 5 Reactome Pathways | + | ||||
| 1 | GPVI-mediated activation cascade | |||||
| 2 | G beta:gamma signalling through PI3Kgamma | |||||
| 3 | Interleukin-3, 5 and GM-CSF signaling | |||||
| 4 | RAF/MAP kinase cascade | |||||
| 5 | Interleukin receptor SHC signaling | |||||
| WikiPathways | [+] 3 WikiPathways | + | ||||
| 1 | IL-3 Signaling Pathway | |||||
| 2 | Interleukin-2 signaling | |||||
| 3 | Interleukin-3, 5 and GM-CSF signaling | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||||
| REF 2 | ClinicalTrials.gov (NCT03631576) CD123/CLL1 CAR-T Cells for R/R AML | |||||
| REF 3 | ClinicalTrials.gov (NCT02152956) Safety Study of MGD006 in Relapsed/Refractory Acute Myeloid Leukemia (AML) or Intermediate-2/High Risk MDS | |||||
| REF 4 | ClinicalTrials.gov (NCT02772276) Pharmacokinetics of MB-102 and Use of the Non-invasive Optical Renal Function Monitor (ORFM) Device in Subjects With Normal and Impaired Renal Function and a Range of Skin Color Types. U.S. National Institutes of Health. | |||||
| REF 5 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 6 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 7 | ClinicalTrials.gov (NCT03125577) Combination CAR-T Cell Therapy Targeting Hematological Malignancies | |||||
| REF 8 | ClinicalTrials.gov (NCT02937103) A Clinical Research of CD123-Targeted CAR-T in Myeloid Malignancies | |||||
| REF 9 | ClinicalTrials.gov (NCT03556982) CART-123 FOR Relapsed/Refractory Acute Myelocytic LeukemiaAML | |||||
| REF 10 | ClinicalTrials.gov (NCT03222674) Multi-CAR T Cell Therapy for Acute Myeloid Leukemia | |||||
| REF 11 | ClinicalTrials.gov (NCT03594955) First in Human Testing of Dose-escalation of SAR440234 in Patients With Acute Myeloid Leukemia, Acute Lymphoid Leukemia and Myelodysplastic Syndrome. U.S. National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT03121625) CAR-T Therapy in Relapsed or Refractory Haematopoietic and Lymphoid Malignancies | |||||
| REF 13 | ClinicalTrials.gov (NCT03647800) Study of APVO436 in Patients With AML or MDS. U.S. National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT03291444) CAR-T Cells Combined With Peptide Specific Dendritic Cell in Relapsed/Refractory Leukemia/MDS | |||||
| REF 15 | ClinicalTrials.gov (NCT03766126) Lentivirally Redirected CD123 Autologous T Cells in AML | |||||
| REF 16 | ClinicalTrials.gov (NCT04156256) CD123-CD33 cCAR in Patients With Relapsed and/or Refractory, High Risk Hematologic Malignancies. U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT03114670) Donor-derived Anti-CD123-CART Cells for Recurred AML After Allo-HSCT | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031973) | |||||
| REF 19 | ClinicalTrials.gov (NCT04082754) A Clinical Study to Test the Safety, Exposure, and Pharmacodynamic Markers of CSL311 in Subjects With Mild-to-moderate Asthma and in Healthy Volunteers. U.S.National Institutes of Health. | |||||
| REF 20 | ClinicalTrials.gov (NCT03585517) Safety and Efficacy Evaluation of IM23 CAR-T Cells (IM23CAR-T) | |||||
| REF 21 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 22 | A Phase I trial of MGD006 in patients with relapsed acute myeloid leukemia (AML). J Immunother Cancer. 2014; 2(Suppl 3): P87. | |||||
| REF 23 | ClinicalTrials.gov (NCT02623582) CD123 Redirected Autologous T Cells for AML | |||||
| REF 24 | ClinicalTrials.gov (NCT03473457) CAR-T Cells Therapy in Relapsed/Refractory Acute Myeloid Leukemia | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005988) | |||||
| REF 26 | Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019 Aug;18(8):585-608. | |||||
| REF 27 | ClinicalTrials.gov (NCT03672851) Study Evaluating Safety and Efficacy of CAR-T Cells Targeting CD123 in Patients With Acute Leukemia | |||||
| REF 28 | Clinical pipeline report, company report or official report of Aptevo. | |||||
| REF 29 | Clinical pipeline report, company report or official report of iCell Gene Therapeutics. | |||||
| REF 30 | Monoclonal antibody targeting of IL-3 receptor alpha with CSL362 effectively depletes CML progenitor and stem cells. Blood. 2014 Feb 20;123(8):1218-28. | |||||
| REF 31 | Anti-beta(c) mAb CSL311 inhibits human nasal polyp pathophysiology in a humanized mouse xenograft model. Allergy. 2020 Feb;75(2):475-478. | |||||
| REF 32 | Immune therapies in acute myeloid leukemia: a focus on monoclonal antibodies and immune checkpoint inhibitors. Curr Opin Hematol. 2018 Mar;25(2):136-145. | |||||
| REF 33 | A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates.Sci Transl Med.2015 May 27;7(289):289ra82. | |||||
| REF 34 | ClinicalTrials.gov (NCT03190278) Study Evaluating Safety and Efficacy of UCART123 in Patients With Acute Myeloid Leukemia | |||||
| REF 35 | ClinicalTrials.gov (NCT03203369) Study to Evaluate the Safety and Clinical Activity of UCART123 in Patients With BPDCN | |||||
| REF 36 | The combined administration of daniplestim and Mpl ligand augments the hematopoietic reconstitution observed with single cytokine administration in a nonhuman primate model of myelosuppression. Stem Cells. 1998;16 Suppl 2:143-54. | |||||
| REF 37 | A dual role for the N-terminal domain of the IL-3 receptor in cell signalling. Nat Commun. 2018 Jan 26;9(1):386. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

