Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T65883
(Former ID: TTDI02045)
|
|||||
| Target Name |
Claudin-18 (CLDN18)
|
|||||
| Synonyms |
UNQ778/PRO1572; Claudin18
Click to Show/Hide
|
|||||
| Gene Name |
CLDN18
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Oesophagogastric junction cancer [ICD-11: 2B71] | |||||
| 2 | Stomach cancer [ICD-11: 2B72] | |||||
| Function |
Plays a major role in tight junction-specific obliteration of the intercellular space, through calcium-independent cell-adhesion activity.
Click to Show/Hide
|
|||||
| UniProt ID | ||||||
| Sequence |
MSTTTCQVVAFLLSILGLAGCIAATGMDMWSTQDLYDNPVTSVFQYEGLWRSCVRQSSGF
TECRPYFTILGLPAMLQAVRALMIVGIVLGAIGLLVSIFALKCIRIGSMEDSAKANMTLT SGIMFIVSGLCAIAGVSVFANMLVTNFWMSTANMYTGMGGMVQTVQTRYTFGAALFVGWV AGGLTLIGGVMMCIACRGLAPEETNYKAVSYHASGHSVAYKPGGFKASTGFGSNTKNKKI YDGGARTEDEVQSYPSKHDYV Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 9 Clinical Trial Drugs | + | ||||
| 1 | Claudiximab | Drug Info | Phase 3 | Gastric adenocarcinoma | [2] | |
| 2 | Zolbetuximab | Drug Info | Phase 3 | Gastric adenocarcinoma | [3] | |
| 3 | CT-041 | Drug Info | Phase 1/2 | Gastric cancer | [4] | |
| 4 | RC118 | Drug Info | Phase 1/2 | Aggressive cancer | [5] | |
| 5 | AMG 910 | Drug Info | Phase 1 | Gastric adenocarcinoma | [6] | |
| 6 | ASP2138 | Drug Info | Phase 1 | Stomach cancer | [7] | |
| 7 | SKB315 | Drug Info | Phase 1 | Aggressive cancer | [8] | |
| 8 | TST001 | Drug Info | Phase 1 | Solid tumour/cancer | [9] | |
| 9 | CAR-CLD18 T cell | Drug Info | Clinical trial | Adenocarcinoma | [10] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | BNT212 | Drug Info | Preclinical | Aggressive cancer | [11] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 1 Inhibitor drugs | + | ||||
| 1 | AMG 910 | Drug Info | [14] | |||
| CAR-T-Cell-Therapy | [+] 1 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | CAR-CLD18 T cell | Drug Info | [10] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cell adhesion molecules | hsa04514 | Affiliated Target |
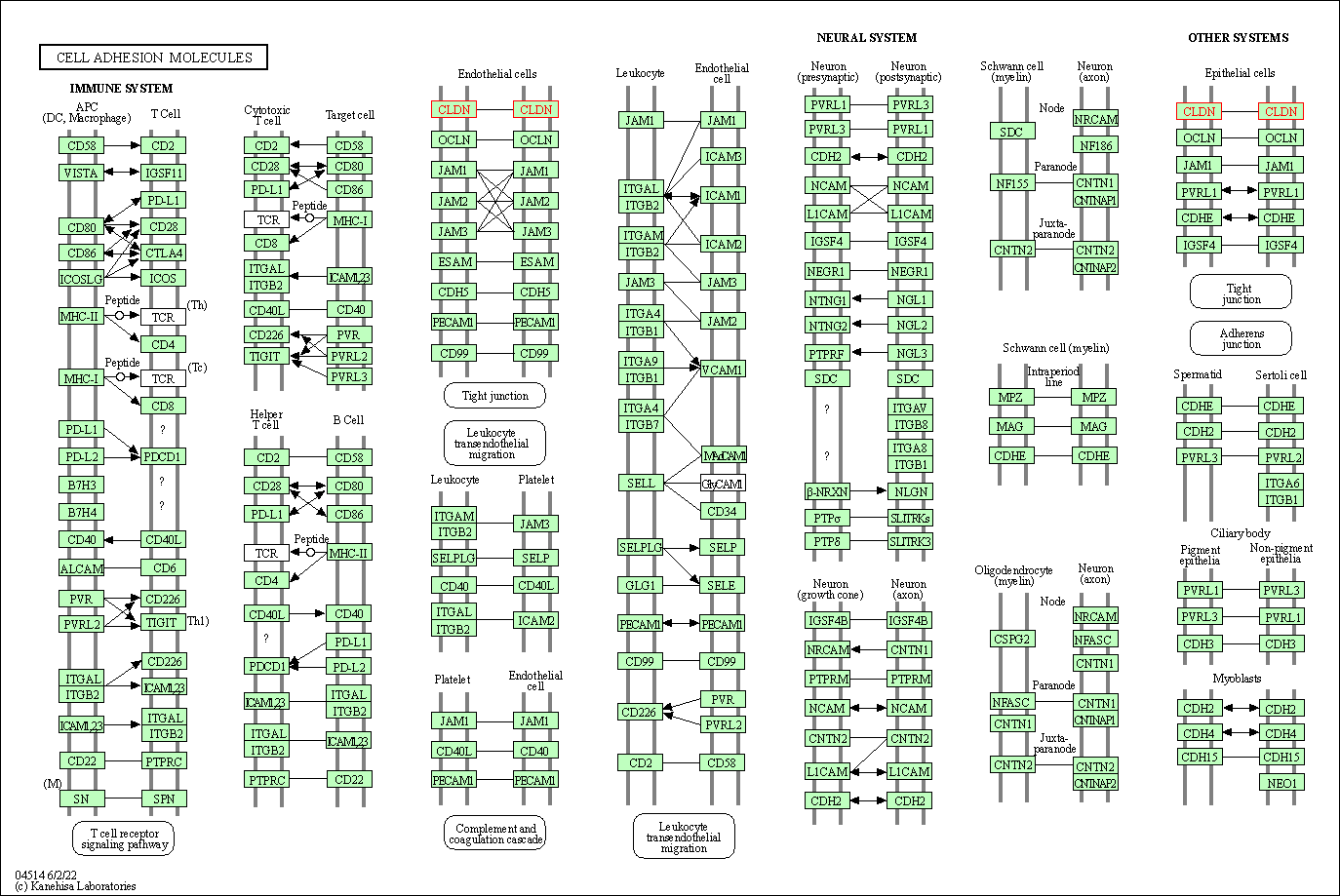
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Tight junction | hsa04530 | Affiliated Target |
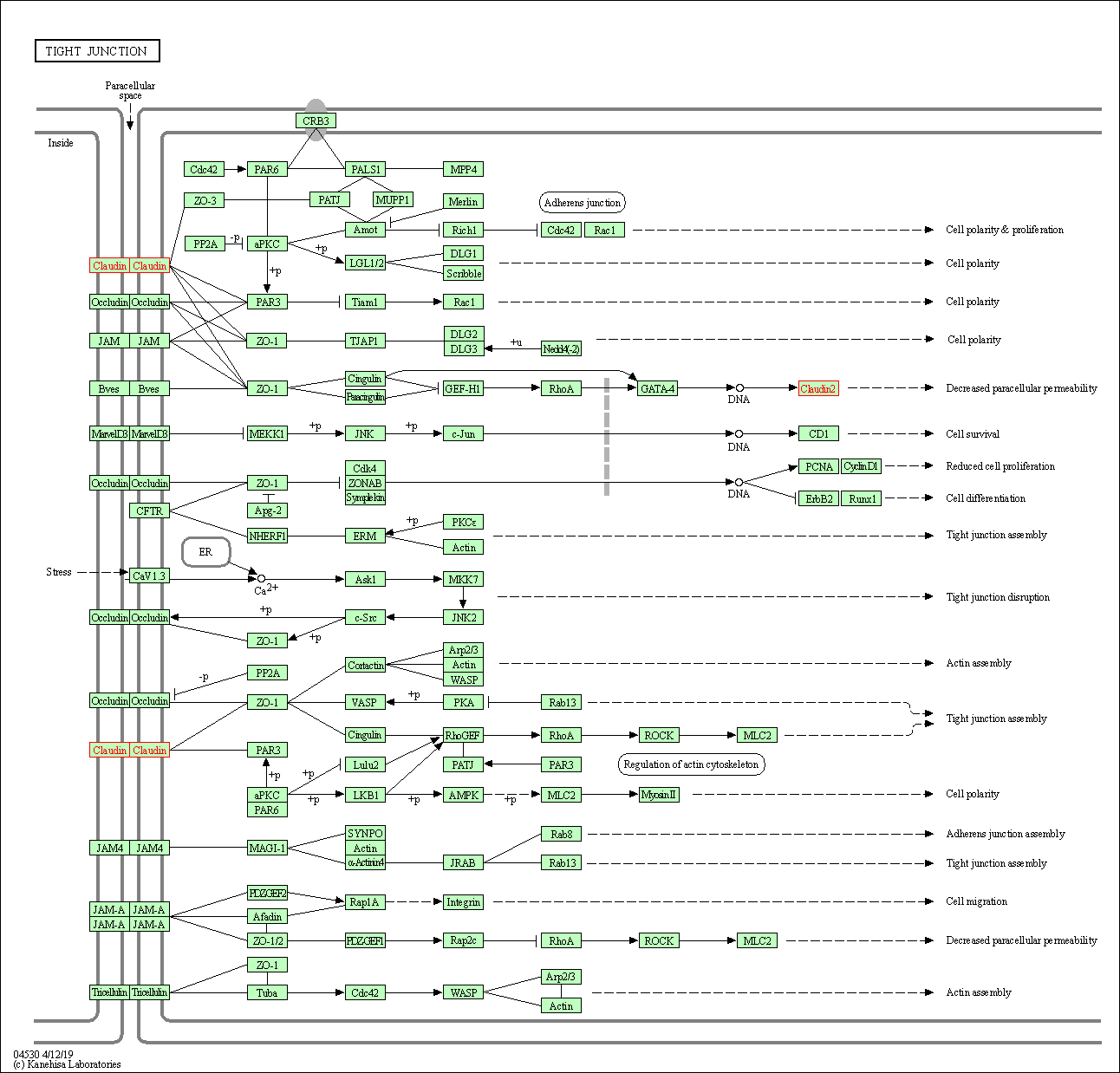
|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Leukocyte transendothelial migration | hsa04670 | Affiliated Target |
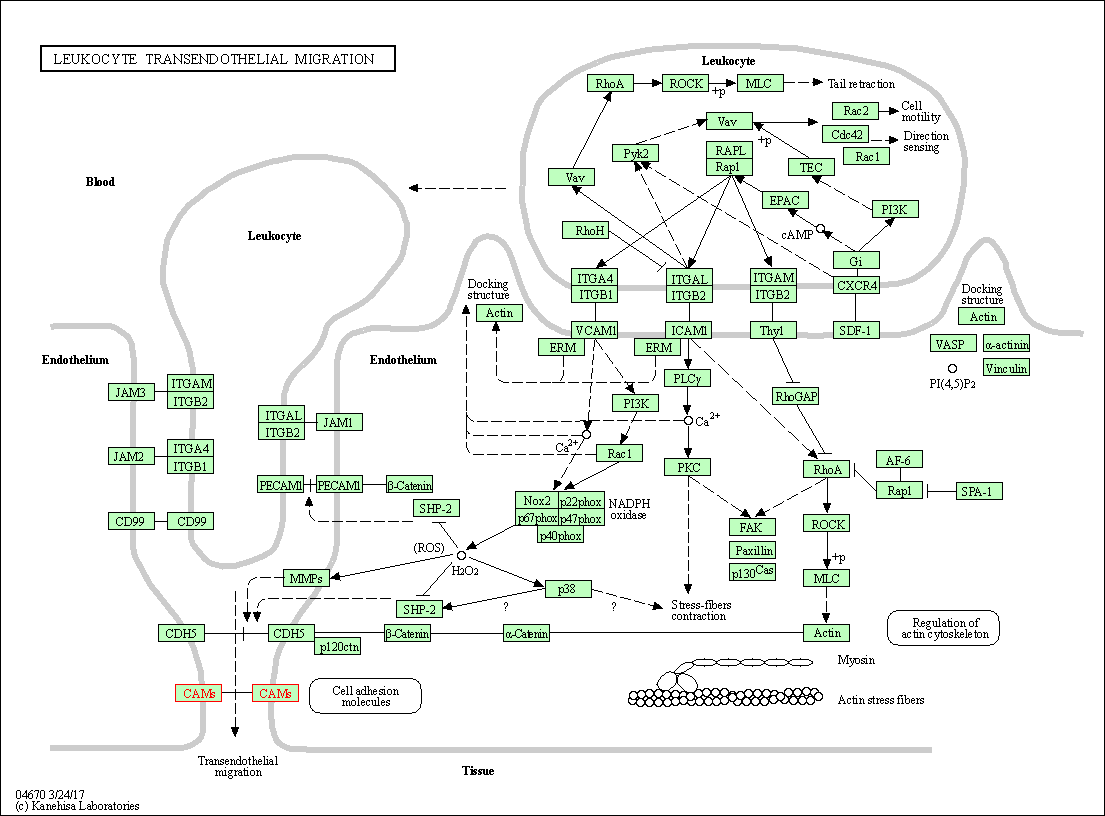
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 4 KEGG Pathways | + | ||||
| 1 | Cell adhesion molecules (CAMs) | |||||
| 2 | Tight junction | |||||
| 3 | Leukocyte transendothelial migration | |||||
| 4 | Hepatitis C | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | IL4 Signaling Pathway | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | Tight junction interactions | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer. 2014 Feb 1;134(3):731-9. | |||||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 3 | ClinicalTrials.gov (NCT03504397) A Phase 3 Efficacy, Safety and Tolerability Study of Zolbetuximab (Experimental Drug) Plus mFOLFOX6 Chemotherapy Compared to Placebo Plus mFOLFOX6 as Treatment for Gastric and Gastroesophageal Junction (GEJ) Cancer (Spotlight). U.S. National Institutes of Health. | |||||
| REF 4 | ClinicalTrials.gov (NCT04404595) Open-label, Multicenter, Phase 1b/2 Clinical Trial to Evaluate the Safety and Efficacy of Autologous Anti-claudin 18.2 Chimeric Antigen Receptor T-cell Therapy in Subjects With Advanced Gastric, Pancreatic, or Other Specified Digestive System Cancers. U.S.National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT05205850) An Open, Multi-center Phase I/IIa Clinical Study of RC118 for Injection in Patients With Locally Advanced Unresectable or Metastatic Malignant Solid Tumors With Positive Expression of Claudin 18.2. U.S.National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT04260191) Study of AMG 910 in Subjects With CLDN18.2-Positive Gastric and Gastroesophageal Junction Adenocarcinoma. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT05365581) A Phase 1/1b Study of ASP2138 in Participants With Metastatic or Locally Advanced Unresectable Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma or Metastatic Pancreatic Adenocarcinoma Whose Tumors Have Claudin (CLDN) 18.2 Expression. U.S.National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT05367635) A Phase 1 Clinical Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of SKB315 for Injection in Patients With Advanced Solid Tumors Expressing Claudin18.2. U.S.National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT04495296) A Trial to Evaluate Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of TST001 in Advanced or Metastatic Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT03302403) Clinical Study of Redirected Autologous T Cells With a Chimeric Antigen Receptor in Patients With Malignant Tumors | |||||
| REF 11 | Clinical pipeline report, company report or official report of Biontech | |||||
| REF 12 | FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021 May;32(5):609-619. | |||||
| REF 13 | Clinical pipeline report, company report or official report of CARSGEN | |||||
| REF 14 | Clinical pipeline report, company report or official report of Amgen. | |||||
| REF 15 | Clinical pipeline report, company report or official report of Klus Pharma | |||||
| REF 16 | Clinical pipeline report, company report or official report of Transcenta. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

