Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T46040
(Former ID: TTDI01964)
|
|||||
| Target Name |
Coagulation factor XI (F11)
|
|||||
| Synonyms |
Plasma thromboplastin antecedent; PTA; FXI
Click to Show/Hide
|
|||||
| Gene Name |
F11
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Clotting disorder ICD-11: 3B4Z | |||||
| 2 | Cerebral ischaemic stroke [ICD-11: 8B11] | |||||
| Function |
Factor XI triggers the middle phase of the intrinsic pathway of blood coagulation by activating factor IX.
Click to Show/Hide
|
|||||
| BioChemical Class |
Peptidase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.4.21.27
|
|||||
| Sequence |
MIFLYQVVHFILFTSVSGECVTQLLKDTCFEGGDITTVFTPSAKYCQVVCTYHPRCLLFT
FTAESPSEDPTRWFTCVLKDSVTETLPRVNRTAAISGYSFKQCSHQISACNKDIYVDLDM KGINYNSSVAKSAQECQERCTDDVHCHFFTYATRQFPSLEHRNICLLKHTQTGTPTRITK LDKVVSGFSLKSCALSNLACIRDIFPNTVFADSNIDSVMAPDAFVCGRICTHHPGCLFFT FFSQEWPKESQRNLCLLKTSESGLPSTRIKKSKALSGFSLQSCRHSIPVFCHSSFYHDTD FLGEELDIVAAKSHEACQKLCTNAVRCQFFTYTPAQASCNEGKGKCYLKLSSNGSPTKIL HGRGGISGYTLRLCKMDNECTTKIKPRIVGGTASVRGEWPWQVTLHTTSPTQRHLCGGSI IGNQWILTAAHCFYGVESPKILRVYSGILNQSEIKEDTSFFGVQEIIIHDQYKMAESGYD IALLKLETTVNYTDSQRPICLPSKGDRNVIYTDCWVTGWGYRKLRDKIQNTLQKAKIPLV TNEECQKRYRGHKITHKMICAGYREGGKDACKGDSGGPLSCKHNEVWHLVGITSWGEGCA QRERPGVYTNVVEYVDWILEKTQAV Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 7 Clinical Trial Drugs | + | ||||
| 1 | A186 | Drug Info | IND submitted | Clotting disorder | [2] | |
| 2 | Asundexian | Drug Info | Phase 3 | Ischemic stroke | [3] | |
| 3 | Abelacimab | Drug Info | Phase 2 | Atrial fibrillation | [4] | |
| 4 | MK-2060 | Drug Info | Phase 2 | Thrombosis | [5] | |
| 5 | A336 | Drug Info | Phase 1 | Clotting disorder | [2] | |
| 6 | AB023 | Drug Info | Phase 1 | End-stage renal disease | [6] | |
| 7 | EP-7041 | Drug Info | Phase 1 | Thrombosis | [1] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Inhibitor | [+] 5 Inhibitor drugs | + | ||||
| 1 | Asundexian | Drug Info | [7] | |||
| 2 | Abelacimab | Drug Info | [8] | |||
| 3 | MK-2060 | Drug Info | [9] | |||
| 4 | AB023 | Drug Info | [10] | |||
| 5 | EP-7041 | Drug Info | [1] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Glutathione | Ligand Info | |||||
| Structure Description | Crystal Structure of the Catalytic Domain of the Coagulation Factor XIa in Complex with Benzamidine (S434A-T475A-K437 Mutant) | PDB:1ZHM | ||||
| Method | X-ray diffraction | Resolution | 1.96 Å | Mutation | Yes | [11] |
| PDB Sequence |
IVGGTASVRG
25 EWPWQVTLHT35 TSPTQRHLCG41 GSIIGNQWIL53 TAAHCFYGVE60 SPKILRVYSG 70 ILNQAEIAED80 TSFFGVQEII89 IHDQYKMAES99 GYDIALLKLE109 TTVNYADSQR 119 PICLPSKGDR130 NVIYTDCWVT139 GWGYRKLRDK149 IQNTLQKAKI160 PLVTNEECQK 170 RYRGHKITHK179 MICAGYREGG188 KDACKGDSGG197 PLSCKHNEVW203 HLVGITSWGE 217 GCAQRERPGV227 YTNVVEYVDW237 ILEKTQA
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: BMS-986177 | Ligand Info | |||||
| Structure Description | FACTOR XIA (PICHIA PASTORIS; C500S [C122S]) IN COMPLEX WITH THE INHIBITOR Milvexian (BMS-986177), IUPAC NAME:(6R,10S)-10-{4-[5-chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl]-6- oxopyrimidin-1(6H)-yl}-1-(difluoromethyl)-6-methyl-1,4,7,8,9,10-hexahydro-15,11- (metheno)pyrazolo[4,3-b][1,7]diazacyclotetradecin-5(6H)-one | PDB:7MBO | ||||
| Method | X-ray diffraction | Resolution | 0.92 Å | Mutation | Yes | [12] |
| PDB Sequence |
IVGGTASVRG
25 EWPWQVTLHT35 TSPTQRHLCG43 GSIIGNQWIL53 TAAHCFYGVE61 SPKILRVYSG 69 ILNQSEIKED79 TSFFGVQEII89 IHDQYKMAES99 GYDIALLKLE109 TTVNYTDSQR 119 PISLPSKGDR129 NVIYTDCWVT139 GWGYRKLRDK150 IQNTLQKAKI160 PLVTNEECQK 169 RYRGHKITHK179 MICAGYREGG187 KDACKGDSGG197 PLSCKHNEVW207 HLVGITSWGE 217 GCAQRERPGV227 YTNVVEYVDW237 ILEKTQA
|
|||||
|
|
ARG39
3.314
HIS40
2.448
LEU41
1.933
CYS42
3.290
HIS57
2.254
CYS58
2.615
TYR58B
3.996
PHE58A
4.537
GLY59
4.598
TYR143
2.923
LEU147
2.948
ILE151
3.020
ASP189
2.730
ALA190
2.880
CYS191
2.690
LYS192
2.525
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Complement and coagulation cascades | hsa04610 | Affiliated Target |
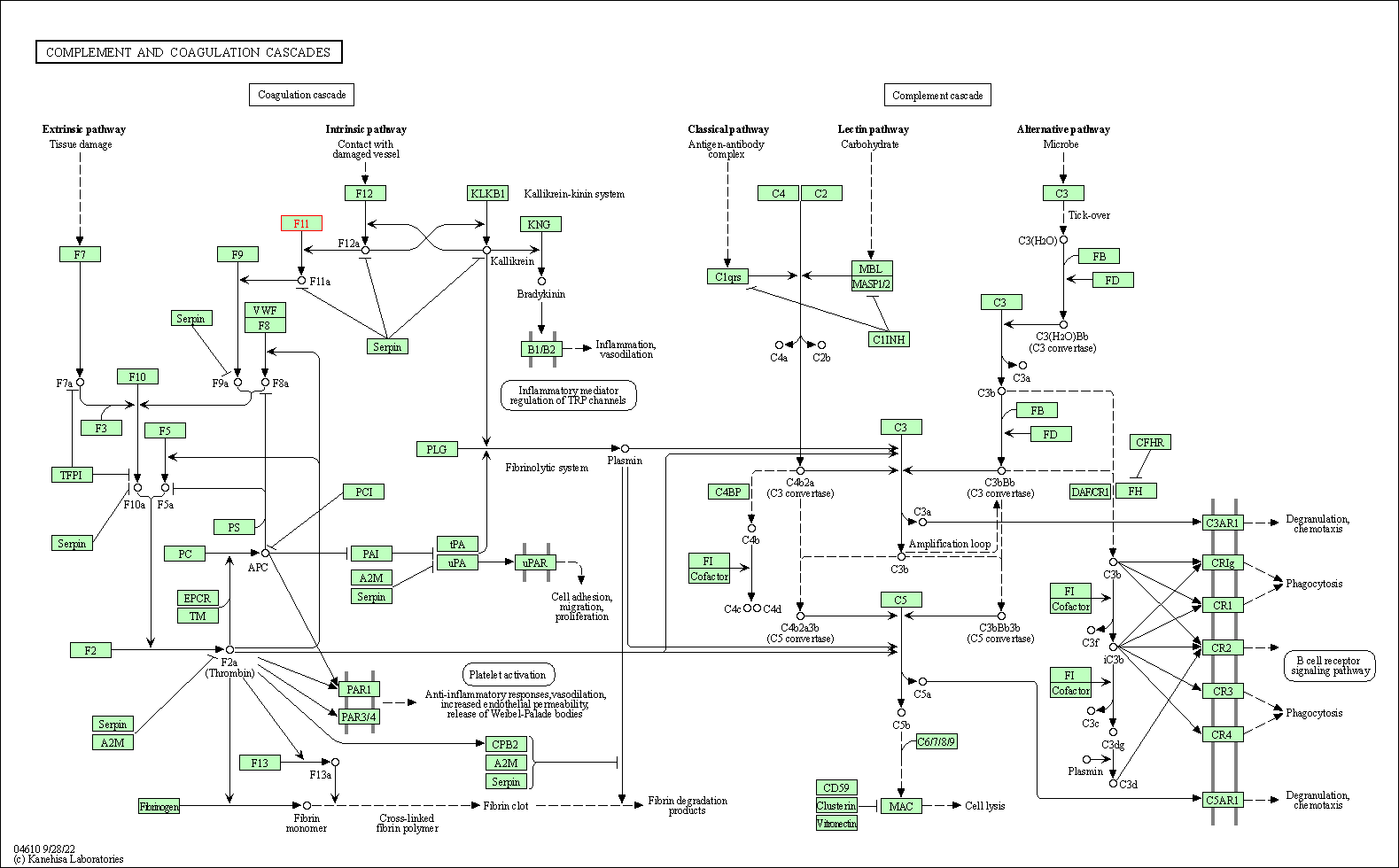
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 9 | Degree centrality | 9.67E-04 | Betweenness centrality | 3.51E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 1.77E-01 | Radiality | 1.29E+01 | Clustering coefficient | 3.61E-01 |
| Neighborhood connectivity | 1.18E+01 | Topological coefficient | 2.26E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 2 | Clinical pipeline report, company report or official report of Klus Pharma | |||||
| REF 3 | ClinicalTrials.gov (NCT05686070) A Multicenter, International, Randomized, Placebo Controlled, Double-blind, Parallel Group and Event Driven Phase 3 Study of the Oral FXIa Inhibitor Asundexian (BAY 2433334) for the Prevention of Ischemic Stroke in Male and Female Participants Aged 18 Years and Older After an Acute Non-cardioembolic Ischemic Stroke or High-risk TIA. U.S.National Institutes of Health. | |||||
| REF 4 | ClinicalTrials.gov (NCT04755283) A Multicenter, RandomiZed, Active-ControLled Study to Evaluate the Safety and Tolerability of Two Blinded Doses of Abelacimab (MAA868) Compared With Open-Label Rivaroxaban in Patients With Atrial Fibrillation (AZALEA). U.S.National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT05027074) A Randomized Parallel-group, Placebo-controlled, Double-blind, Event-driven, Multi-center Phase 2 Clinical Outcome Trial of Prevention of Arteriovenous Graft Thrombosis and Safety of MK-2060 in Patients With End Stage Renal Disease Receiving Hemodialysis. U.S.National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT03097341) Safety and Tolerability Study of Xisomab 3G3 in Healthy Adult Subjects. U.S. National Institutes of Health. | |||||
| REF 7 | Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa. J Thromb Haemost. 2022 Jun;20(6):1400-1411. | |||||
| REF 8 | Pharmacokinetics and pharmacodynamics of Abelacimab (MAA868), a novel dual inhibitor of Factor XI and Factor XIa. J Thromb Haemost. 2022 Feb;20(2):307-315. | |||||
| REF 9 | Clinical pipeline report, company report or official report of MSD | |||||
| REF 10 | Contact Activation Inhibitor and Factor XI Antibody, AB023, Produces Safe, Dose-Dependent Anticoagulation in a Phase 1 First-In-Human Trial. Arterioscler Thromb Vasc Biol. 2019 Apr;39(4):799-809. | |||||
| REF 11 | Mutation of surface residues to promote crystallization of activated factor XI as a complex with benzamidine: an essential step for the iterative structure-based design of factor XI inhibitors. Acta Crystallogr D Biol Crystallogr. 2005 Oct;61(Pt 10):1418-25. | |||||
| REF 12 | Discovery of Milvexian, a High-Affinity, Orally Bioavailable Inhibitor of Factor XIa in Clinical Studies for Antithrombotic Therapy. J Med Chem. 2022 Feb 10;65(3):1770-1785. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

