Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D2HD0V
|
|||
| Drug Name |
Asundexian
|
|||
| Synonyms |
Asundexian; Asundexian [INN]; 2064121-65-7; LA585UM8DE; UNII-LA585UM8DE; 4-[[(2S)-2-[4-[5-chloro-2-[4-(trifluoromethyl)triazol-1-yl]phenyl]-5-methoxy-2-oxopyridin-1-yl]butanoyl]amino]-2-fluorobenzamide; 1(2H)-Pyridineacetamide, N-(4-(aminocarbonyl)-3-fluorophenyl)-4-(5-chloro-2-(4-(trifluoromethyl)-1H-1,2,3-triazol-1-yl)phenyl)-alpha-ethyl-5-methoxy-2-oxo-, (alphaS)-; 4-[[(2~{S})-2-[4-[5-chloranyl-2-[4-(trifluoromethyl)-1,2,3-triazol-1-yl]phenyl]-5-methoxy-2-oxidanylidene-pyridin-1-yl]butanoyl]amino]-2-fluoranyl-benzamide; Asundexian [WHO-DD]; SCHEMBL20360602; GTPL11710; XYWIPYBIIRTJMM-IBGZPJMESA-N; BDBM413842; GLXC-25353; EX-A6019; US10421742, Example 235; AKOS040757232; BAY2433334; BAY 2433334; BAY-2433334; MS-30546; example 235 [WO2017005725A1]; HY-137431; CS-0138630; 1(2H)-PYRIDINEACETAMIDE, N-(4-(AMINOCARBONYL)-3-FLUOROPHENYL)-4-(5-CHLORO-2-(4-(TRIFLUOROMETHYL)-1H-1,2,3-TRIAZOL-1-YL)PHENYL)-.ALPHA.-ETHYL-5-METHOXY-2-OXO-, (.ALPHA.S)-; 4-({(2S)-2-[4-{5-Chloro-2-[4-(trifluoromethyl)-1H-1,2,3-triazol-1-yl]phenyl}-5-methoxy-2-oxopyridin-1(2H)-yl]butanoyl}amino)-2-fluorobenzamide (Enantiomer 2); 4-[(2S)-2-(4-{5-chloro-2-[4-(trifluoromethyl)-1,2,3-triazol-1-yl]phenyl}-5-methoxy-2-oxopyridin-1-yl)butanamido]-2-fluorobenzamide; QV3
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Ischemic stroke [ICD-11: 8B11.5Z; ICD-9: 434.91] | Phase 3 | [1] | |
| Company |
Bayer
|
|||
| Structure |
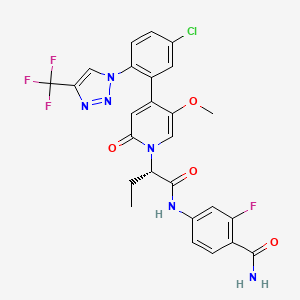 |
Download2D MOL |
||
| Formula |
C26H21ClF4N6O4
|
|||
| Canonical SMILES |
CCC(C(=O)NC1=CC(=C(C=C1)C(=O)N)F)N2C=C(C(=CC2=O)C3=C(C=CC(=C3)Cl)N4C=C(N=N4)C(F)(F)F)OC
|
|||
| InChI |
InChI=1S/C26H21ClF4N6O4/c1-3-19(25(40)33-14-5-6-15(24(32)39)18(28)9-14)36-11-21(41-2)17(10-23(36)38)16-8-13(27)4-7-20(16)37-12-22(34-35-37)26(29,30)31/h4-12,19H,3H2,1-2H3,(H2,32,39)(H,33,40)/t19-/m0/s1
|
|||
| InChIKey |
XYWIPYBIIRTJMM-IBGZPJMESA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Coagulation factor XI (F11) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05686070) A Multicenter, International, Randomized, Placebo Controlled, Double-blind, Parallel Group and Event Driven Phase 3 Study of the Oral FXIa Inhibitor Asundexian (BAY 2433334) for the Prevention of Ischemic Stroke in Male and Female Participants Aged 18 Years and Older After an Acute Non-cardioembolic Ischemic Stroke or High-risk TIA. U.S.National Institutes of Health. | |||
| REF 2 | Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa. J Thromb Haemost. 2022 Jun;20(6):1400-1411. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

