Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T20178
(Former ID: TTDS00131)
|
|||||
| Target Name |
Tumor necrosis factor (TNF)
|
|||||
| Synonyms |
Tumour necrosis factor alpha; Tumour necrosis factor; Tumor necrosis factor ligand superfamily member 2; TNFalpha; TNFSF2; TNFA; TNF-alpha; TNF-a; TNF alpha; Cachectin
Click to Show/Hide
|
|||||
| Gene Name |
TNF
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 5 Target-related Diseases | + | ||||
| 1 | Arterial occlusive disease [ICD-11: BD40] | |||||
| 2 | Multiple myeloma [ICD-11: 2A83] | |||||
| 3 | Pancreatitis [ICD-11: DC31-DC34] | |||||
| 4 | Psoriasis [ICD-11: EA90] | |||||
| 5 | Rheumatoid arthritis [ICD-11: FA20] | |||||
| Function |
It is mainly secreted by macrophages and can induce cell death of certain tumor cell lines. It is potent pyrogen causing fever by direct action or by stimulation of interleukin-1 secretion and is implicated in the induction of cachexia, Under certain conditions it can stimulate cell proliferation and induce cell differentiation. Impairs regulatory T-cells (Treg) function in individuals with rheumatoid arthritis via FOXP3 dephosphorylation. Upregulates the expression of protein phosphatase 1 (PP1), which dephosphorylates the key 'Ser-418' residue of FOXP3, thereby inactivating FOXP3 and rendering Treg cells functionally defective. Key mediator of cell death in the anticancer action of BCG-stimulated neutrophils in combination with DIABLO/SMAC mimetic in the RT4v6 bladder cancer cell line. Induces insulin resistance in adipocytes via inhibition of insulin-induced IRS1 tyrosine phosphorylation and insulin-induced glucose uptake. Induces GKAP42 protein degradation in adipocytes which is partially responsible for TNF-induced insulin resistance. Cytokine that binds to TNFRSF1A/TNFR1 and TNFRSF1B/TNFBR.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine: tumor necrosis factor
|
|||||
| UniProt ID | ||||||
| Sequence |
MSTESMIRDVELAEEALPKKTGGPQGSRRCLFLSLFSFLIVAGATTLFCLLHFGVIGPQR
EEFPRDLSLISPLAQAVRSSSRTPSDKPVAHVVANPQAEGQLQWLNRRANALLANGVELR DNQLVVPSEGLYLIYSQVLFKGQGCPSTHVLLTHTISRIAVSYQTKVNLLSAIKSPCQRE TPEGAEAKPWYEPIYLGGVFQLEKGDRLSAEINRPDYLDFAESGQVYFGIIAL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A00402 ; BADD_A02863 ; BADD_A03065 ; BADD_A03633 ; BADD_A04357 ; BADD_A05322 ; BADD_A05438 ; BADD_A05639 ; BADD_A05823 ; BADD_A05962 | |||||
| HIT2.0 ID | T23VMI | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 10 Approved Drugs | + | ||||
| 1 | Adalimumab | Drug Info | Approved | Rheumatoid arthritis | [2], [3] | |
| 2 | Certolizumab | Drug Info | Approved | Rheumatoid arthritis | [4] | |
| 3 | Enbrel | Drug Info | Approved | Arthritis | [5] | |
| 4 | Etanercept | Drug Info | Approved | Arthritis | [6] | |
| 5 | Golimumab | Drug Info | Approved | Rheumatoid arthritis | [7] | |
| 6 | Infliximab | Drug Info | Approved | Plaque psoriasis | [8], [9], [10] | |
| 7 | Lenalidomide | Drug Info | Approved | Multiple myeloma | [11], [12], [13], [14] | |
| 8 | Nafamostat | Drug Info | Approved | Pancreatitis | [15], [14] | |
| 9 | Pentoxifylline | Drug Info | Approved | Intermittent claudication | [16], [17] | |
| 10 | Thalidomide | Drug Info | Approved | Multiple myeloma | [18], [19] | |
| Clinical Trial Drug(s) | [+] 31 Clinical Trial Drugs | + | ||||
| 1 | ABP 501 | Drug Info | Phase 3 | Rheumatoid arthritis | [20] | |
| 2 | AVT02 | Drug Info | Phase 3 | Plaque psoriasis | [21] | |
| 3 | CPL-7075 | Drug Info | Phase 3 | Diffuse systemic sclerosis | [22] | |
| 4 | Golnerminogene pradenovac | Drug Info | Phase 3 | Esophageal cancer | [23] | |
| 5 | PF-06410293 | Drug Info | Phase 3 | Rheumatoid arthritis | [24] | |
| 6 | PF-06438179 | Drug Info | Phase 3 | Rheumatoid arthritis | [24] | |
| 7 | ABBV-154 | Drug Info | Phase 2 | Crohn disease | [25] | |
| 8 | ABT-122 | Drug Info | Phase 2 | Rheumatoid arthritis | [26], [27], [24] | |
| 9 | AN0128 | Drug Info | Phase 2 | Psoriatic disorder | [28] | |
| 10 | AP-301-IH | Drug Info | Phase 2 | Pneumonia | [29] | |
| 11 | BAICALEIN | Drug Info | Phase 2 | Influenza virus infection | [30] | |
| 12 | DLX-105 | Drug Info | Phase 2 | Osteoarthritis | [31] | |
| 13 | ESBA-105 | Drug Info | Phase 2 | Ocular disease | [32] | |
| 14 | Ortataxel | Drug Info | Phase 2 | Solid tumour/cancer | [33] | |
| 15 | Pegsunercept | Drug Info | Phase 2 | Rheumatoid arthritis | [34] | |
| 16 | RDP58 | Drug Info | Phase 2 | Diarrhea | [35] | |
| 17 | TNF alpha kinoid | Drug Info | Phase 2 | Autoimmune diabetes | [36] | |
| 18 | COVA322 | Drug Info | Phase 1/2a | Psoriasis vulgaris | [37] | |
| 19 | ART621 | Drug Info | Phase 1/2 | Psoriasis vulgaris | [38] | |
| 20 | XTMAB-16 | Drug Info | Phase 1/2 | Sarcoidosis | [39] | |
| 21 | ABBV-257 | Drug Info | Phase 1 | Rheumatoid arthritis | [24] | |
| 22 | AST-005 | Drug Info | Phase 1 | Psoriasis vulgaris | [40], [24] | |
| 23 | AVX-470 | Drug Info | Phase 1 | Inflammatory bowel disease | [41] | |
| 24 | CYT-609 | Drug Info | Phase 1 | Solid tumour/cancer | [42] | |
| 25 | IA-14069 | Drug Info | Phase 1 | Rheumatoid arthritis | [43] | |
| 26 | INB03 | Drug Info | Phase 1 | Solid tumour/cancer | [44] | |
| 27 | PF-05230905 | Drug Info | Phase 1 | Rheumatoid arthritis | [45] | |
| 28 | PMI-005 | Drug Info | Phase 1 | Osteoarthritis | [46] | |
| 29 | SAR441566 | Drug Info | Phase 1 | Inflammation | [47] | |
| 30 | SAR442970 | Drug Info | Phase 1 | Inflammation | [48] | |
| 31 | SAR444419 | Drug Info | Phase 1 | Inflammation | [48] | |
| Discontinued Drug(s) | [+] 10 Discontinued Drugs | + | ||||
| 1 | CDP571 | Drug Info | Discontinued in Phase 3 | Crohn disease | [49] | |
| 2 | Segard | Drug Info | Discontinued in Phase 3 | Sepsis | [50] | |
| 3 | Camobucol | Drug Info | Discontinued in Phase 2 | Rheumatoid arthritis | [51] | |
| 4 | CRx-191 | Drug Info | Discontinued in Phase 2 | Psoriatic disorder | [52] | |
| 5 | AME-527 | Drug Info | Discontinued in Phase 1/2 | Rheumatoid arthritis | [53] | |
| 6 | CYT-007-TNFQb | Drug Info | Discontinued in Phase 1/2 | Allergic rhinitis | [54] | |
| 7 | ALS-00T2-0501 | Drug Info | Discontinued in Phase 1 | Psoriasis vulgaris | [55] | |
| 8 | FR-133605 | Drug Info | Terminated | Arthritis | [58] | |
| 9 | MDL-201112 | Drug Info | Terminated | Colitis | [59] | |
| 10 | TNFQb therapeutic vaccines | Drug Info | Terminated | Crohn disease | [60] | |
| Preclinical Drug(s) | [+] 2 Preclinical Drugs | + | ||||
| 1 | ABX-0401 | Drug Info | Preclinical | Inflammatory bowel disease | [56] | |
| 2 | Celastrol | Drug Info | Preclinical | Motor neurone disease | [57] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Inhibitor | [+] 30 Inhibitor drugs | + | ||||
| 1 | Certolizumab | Drug Info | [4] | |||
| 2 | Lenalidomide | Drug Info | [64], [65] | |||
| 3 | Thalidomide | Drug Info | [69] | |||
| 4 | CPL-7075 | Drug Info | [72] | |||
| 5 | PF-06438179 | Drug Info | [75] | |||
| 6 | AN0128 | Drug Info | [78], [79], [80] | |||
| 7 | AP-301-IH | Drug Info | [81] | |||
| 8 | BAICALEIN | Drug Info | [82] | |||
| 9 | Ortataxel | Drug Info | [85] | |||
| 10 | Pegsunercept | Drug Info | [86] | |||
| 11 | RDP58 | Drug Info | [35] | |||
| 12 | ART621 | Drug Info | [38] | |||
| 13 | ABBV-257 | Drug Info | [24] | |||
| 14 | AST-005 | Drug Info | [40], [24] | |||
| 15 | AVX-470 | Drug Info | [24] | |||
| 16 | IA-14069 | Drug Info | [91] | |||
| 17 | INB03 | Drug Info | [44] | |||
| 18 | PF-05230905 | Drug Info | [92] | |||
| 19 | PMI-005 | Drug Info | [93] | |||
| 20 | SAR441566 | Drug Info | [94] | |||
| 21 | Camobucol | Drug Info | [97] | |||
| 22 | CRx-191 | Drug Info | [98] | |||
| 23 | CYT-007-TNFQb | Drug Info | [100] | |||
| 24 | ABX-0401 | Drug Info | [102] | |||
| 25 | MDL-201112 | Drug Info | [103] | |||
| 26 | 2-Propanol, Isopropanol | Drug Info | [104] | |||
| 27 | IK-862 | Drug Info | [106] | |||
| 28 | PKF-241-466 | Drug Info | [107] | |||
| 29 | PKF-242-484 | Drug Info | [107] | |||
| 30 | Y-39041 | Drug Info | [108] | |||
| Modulator | [+] 10 Modulator drugs | + | ||||
| 1 | Enbrel | Drug Info | [5] | |||
| 2 | Etanercept | Drug Info | [61], [62] | |||
| 3 | Golnerminogene pradenovac | Drug Info | [73] | |||
| 4 | ABT-122 | Drug Info | [77] | |||
| 5 | COVA322 | Drug Info | [88] | |||
| 6 | CYT-609 | Drug Info | [90] | |||
| 7 | CDP571 | Drug Info | [95] | |||
| 8 | ALS-00T2-0501 | Drug Info | [101] | |||
| 9 | FR-133605 | Drug Info | [58] | |||
| 10 | DOM-0215 | Drug Info | [105] | |||
| Suppressor | [+] 1 Suppressor drugs | + | ||||
| 1 | Celastrol | Drug Info | [57] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: 4-N-benzyl-4-N-[2-[3-(2-piperazin-1-ylpyrimidin-5-yl)phenyl]phenyl]pyrimidine-2,4-diamine | Ligand Info | |||||
| Structure Description | HUMAN TNF-ALPHA IN COMPLEX WITH JNJ525 | PDB:5MU8 | ||||
| Method | X-ray diffraction | Resolution | 3.00 Å | Mutation | No | [109] |
| PDB Sequence |
SDKPVAHVVA
18 NPQAEGQLQW28 LNALLANGVE42 LRDNQLVVPS52 EGLYLIYSQV62 LFKGQGCPST 72 HVLLTHTISR82 IAVSYQTKVN92 LLSAIKSPCQ102 RETEAKPWYE116 PIYLGGVFQL 126 EKGDRLSAEI136 NRPDYLDFAE146 SGQVYFGIIA156 L
|
|||||
|
|
||||||
| Ligand Name: (1-Oxyl-2,2,5,5-tetramethylpyrroline-3-methyl)methanethiosulfonate | Ligand Info | |||||
| Structure Description | Crystal Structure of Spin-Labeled T77C TNFa | PDB:5UUI | ||||
| Method | X-ray diffraction | Resolution | 1.40 Å | Mutation | Yes | [110] |
| PDB Sequence |
SDKPVAHVVA
18 NPQAEGQLQW28 LNRRANALLA38 NGVELRDNQL48 VVPSEGLYLI58 YSQVLFKGQG 68 VLLCHTISRI83 AVSYQTKVNL93 LSAIKSPAKP113 WYEPIYLGGV123 FQLEKGDRLS 133 AEINRPDYLD143 FAESGQVYFG153 IIAL
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
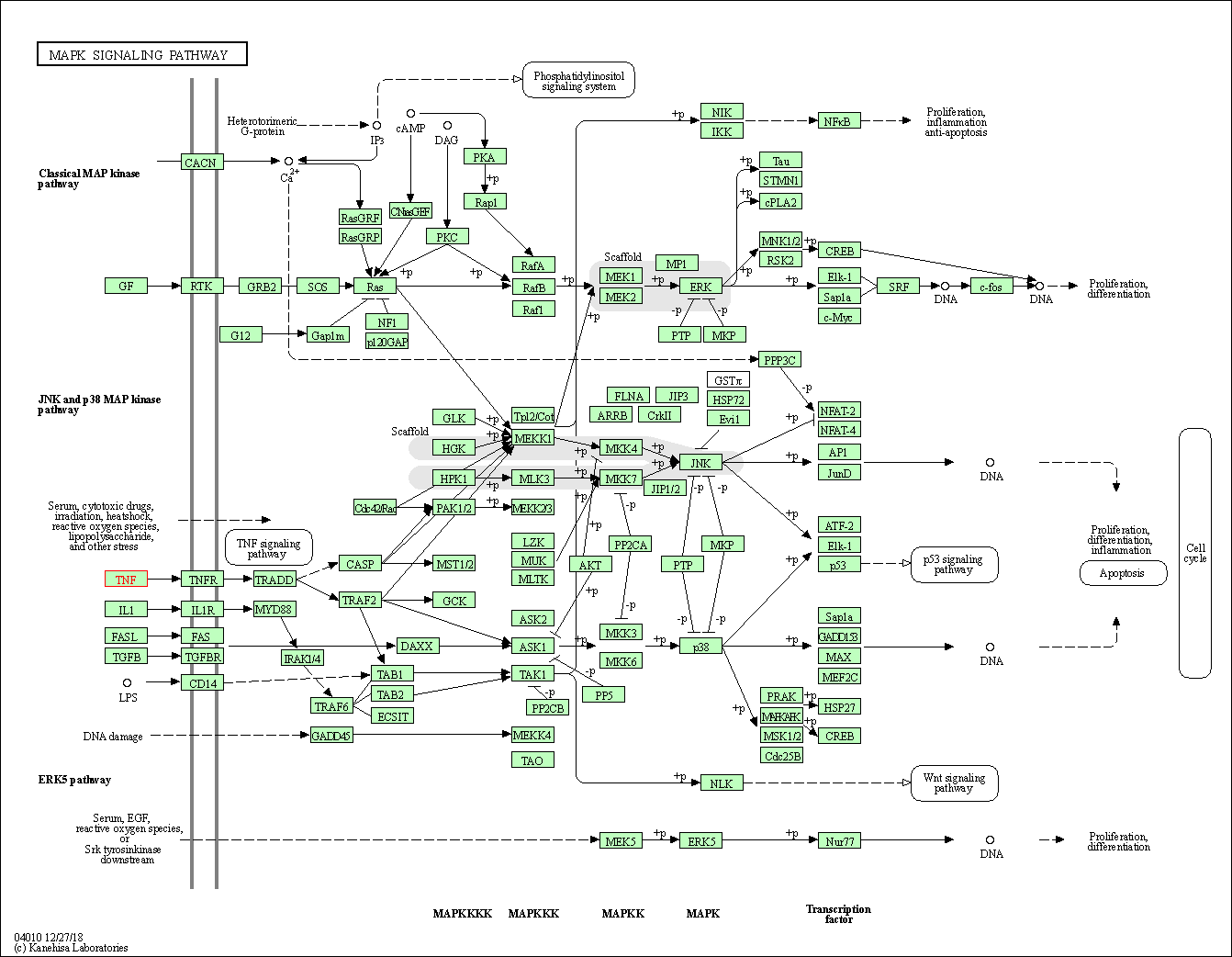
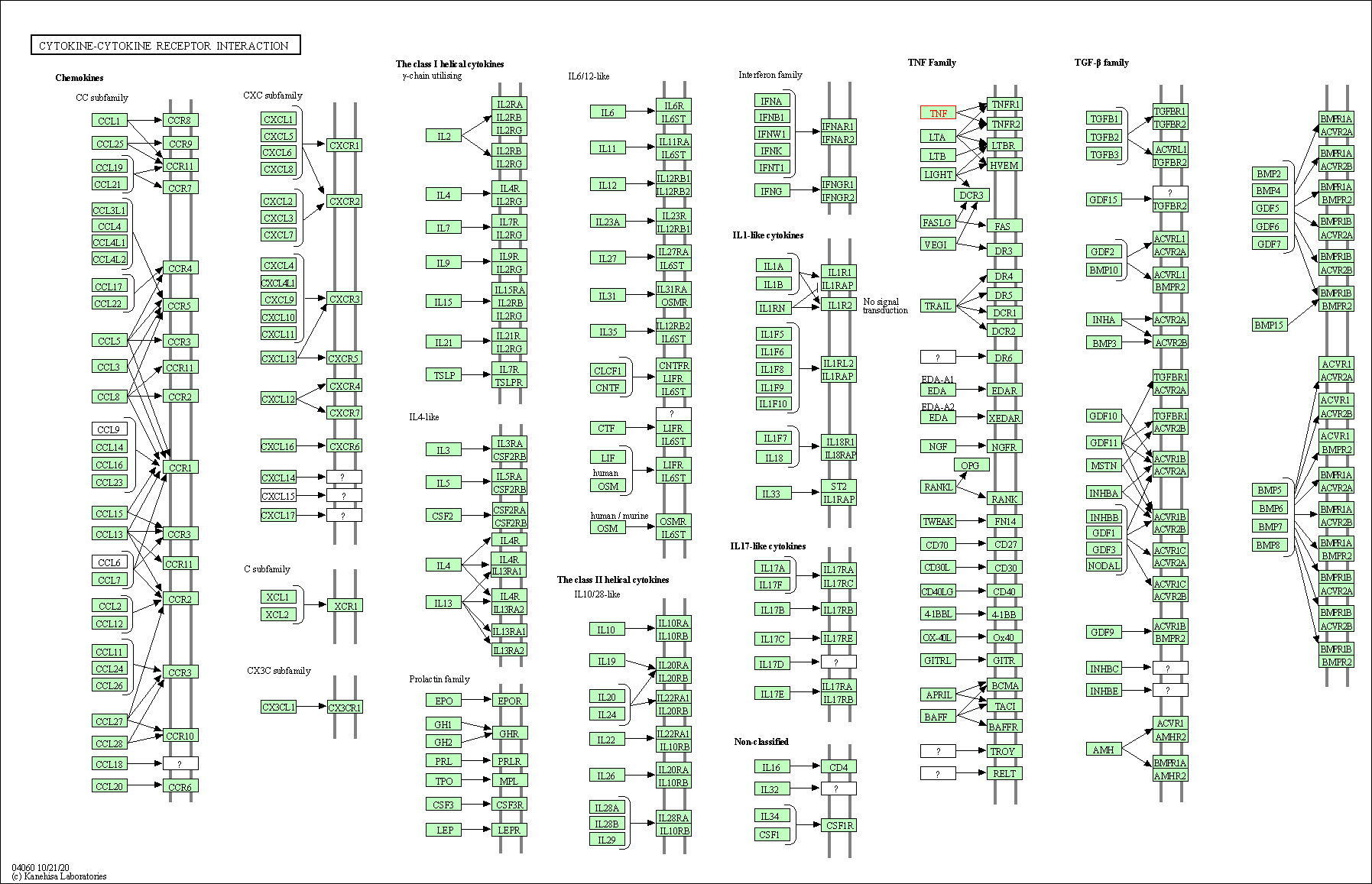
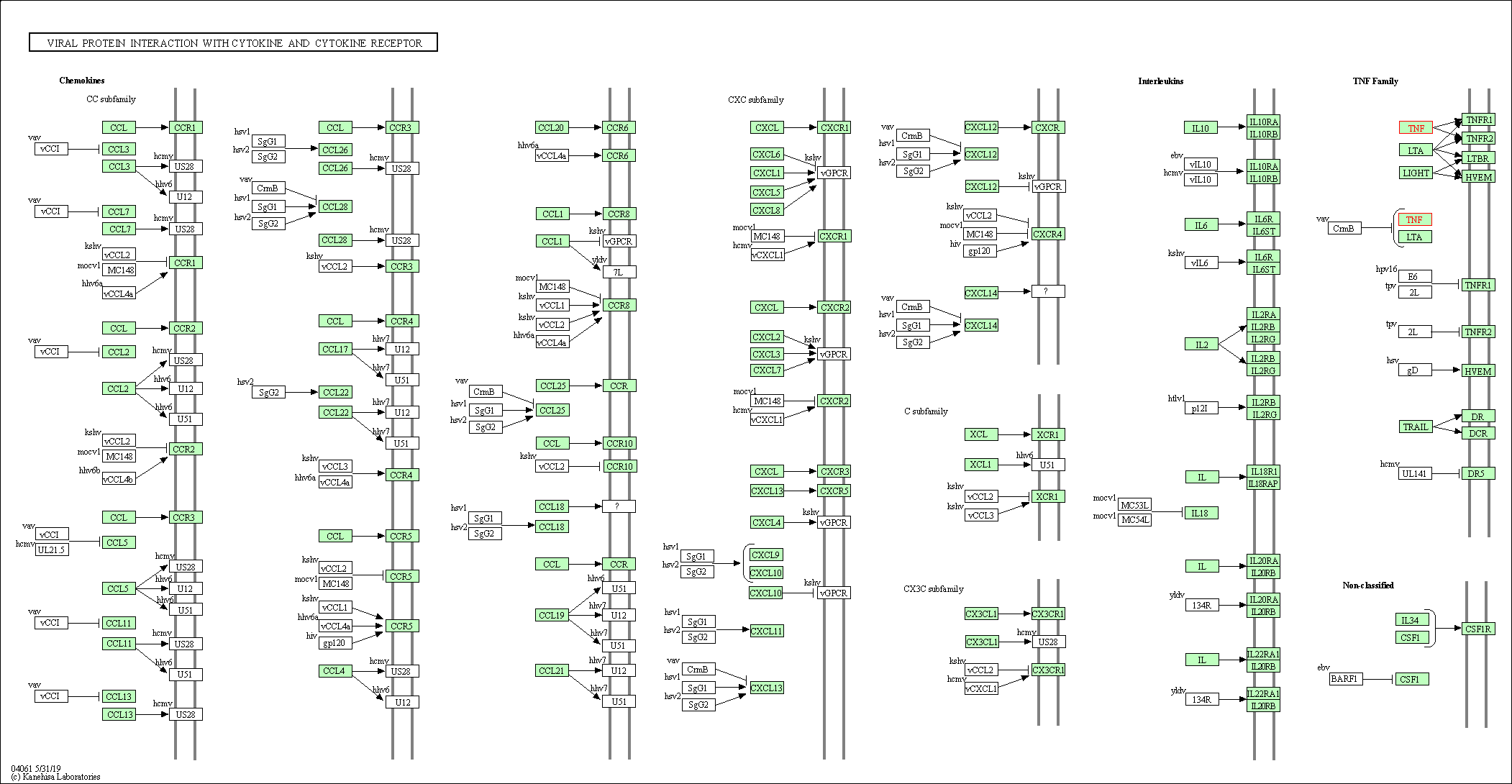
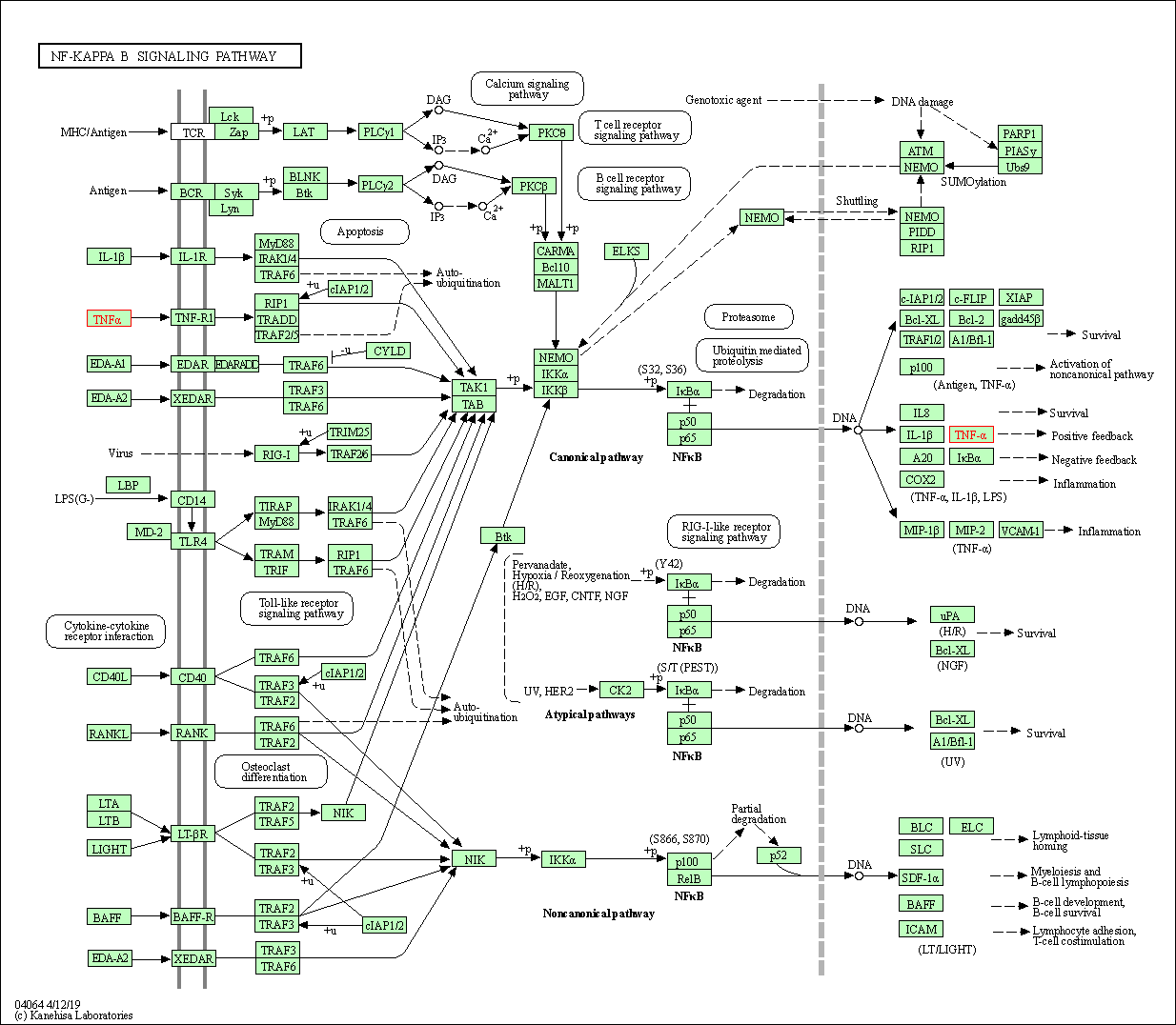
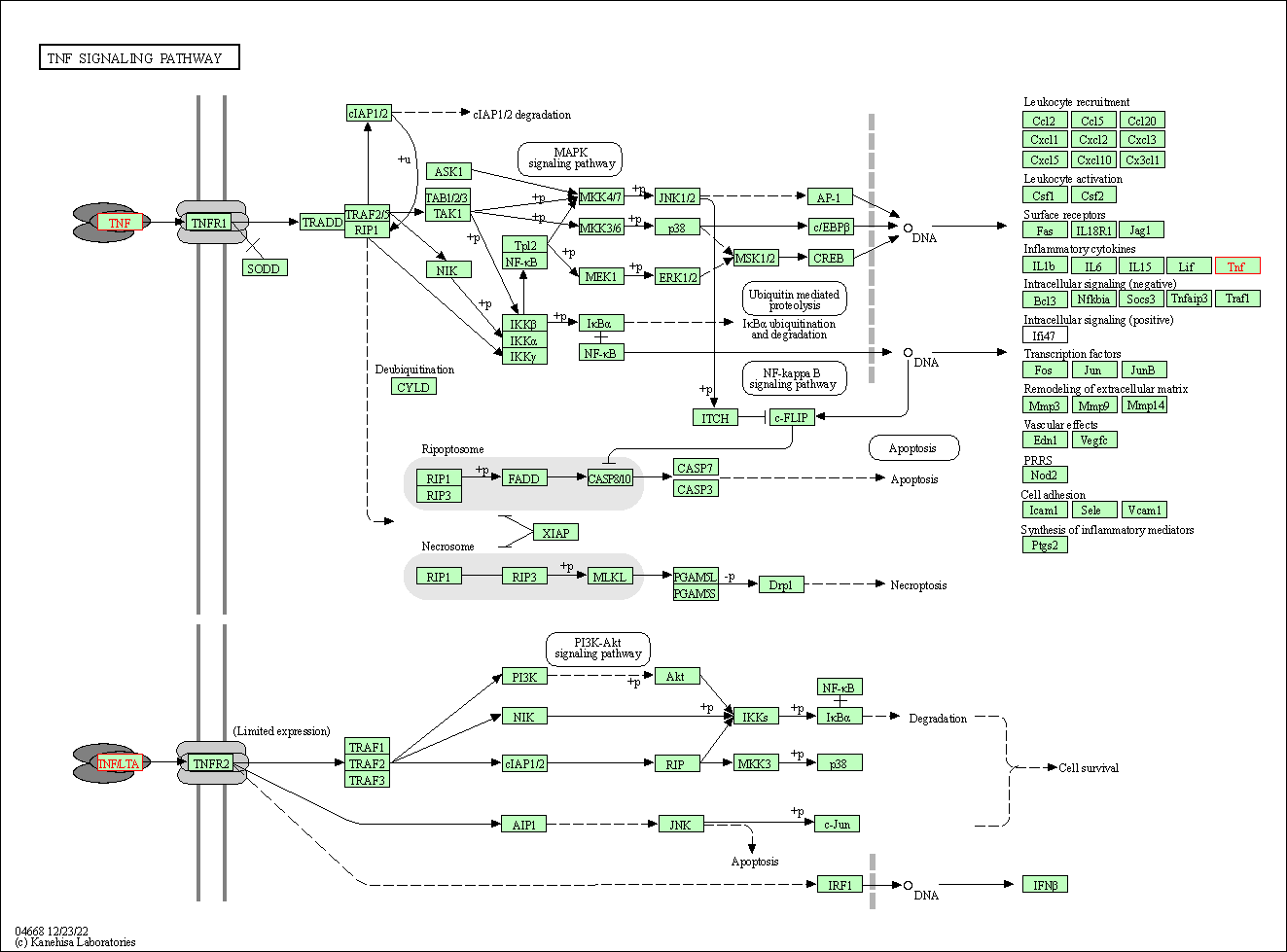
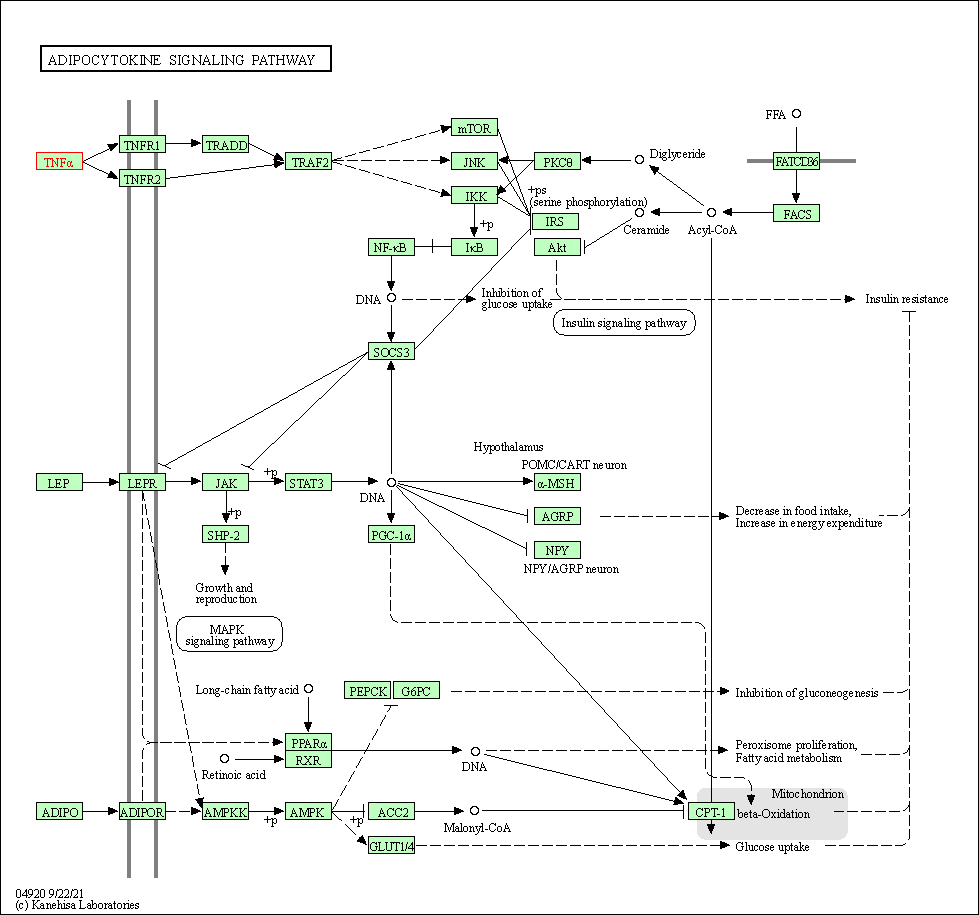
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Viral protein interaction with cytokine and cytokine receptor | hsa04061 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| NF-kappa B signaling pathway | hsa04064 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Sphingolipid signaling pathway | hsa04071 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| mTOR signaling pathway | hsa04150 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Apoptosis | hsa04210 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Necroptosis | hsa04217 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| TGF-beta signaling pathway | hsa04350 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Osteoclast differentiation | hsa04380 | Affiliated Target |

|
| Class: Organismal Systems => Development and regeneration | Pathway Hierarchy | ||
| Antigen processing and presentation | hsa04612 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Toll-like receptor signaling pathway | hsa04620 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
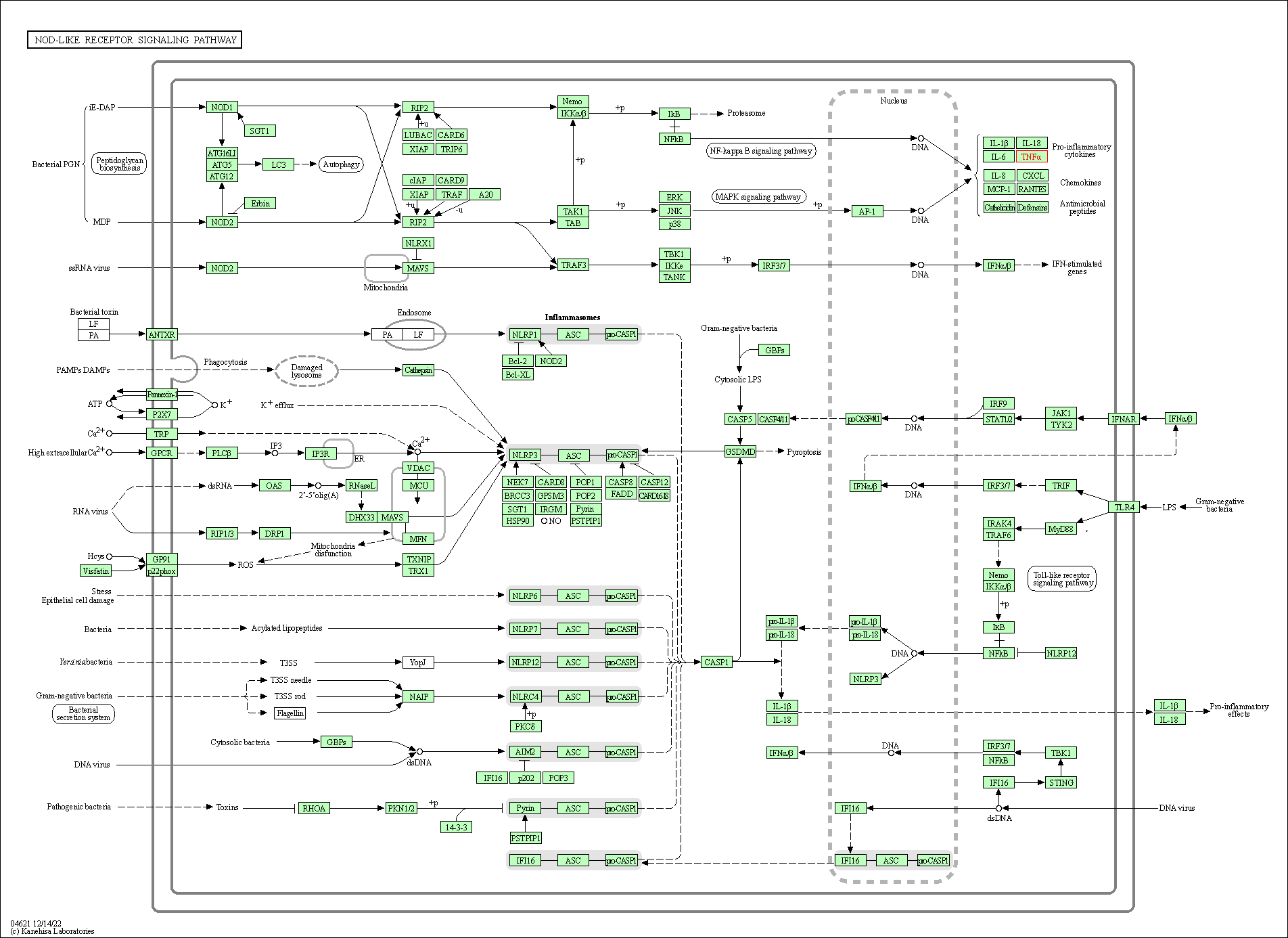
| NOD-like receptor signaling pathway | hsa04621 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
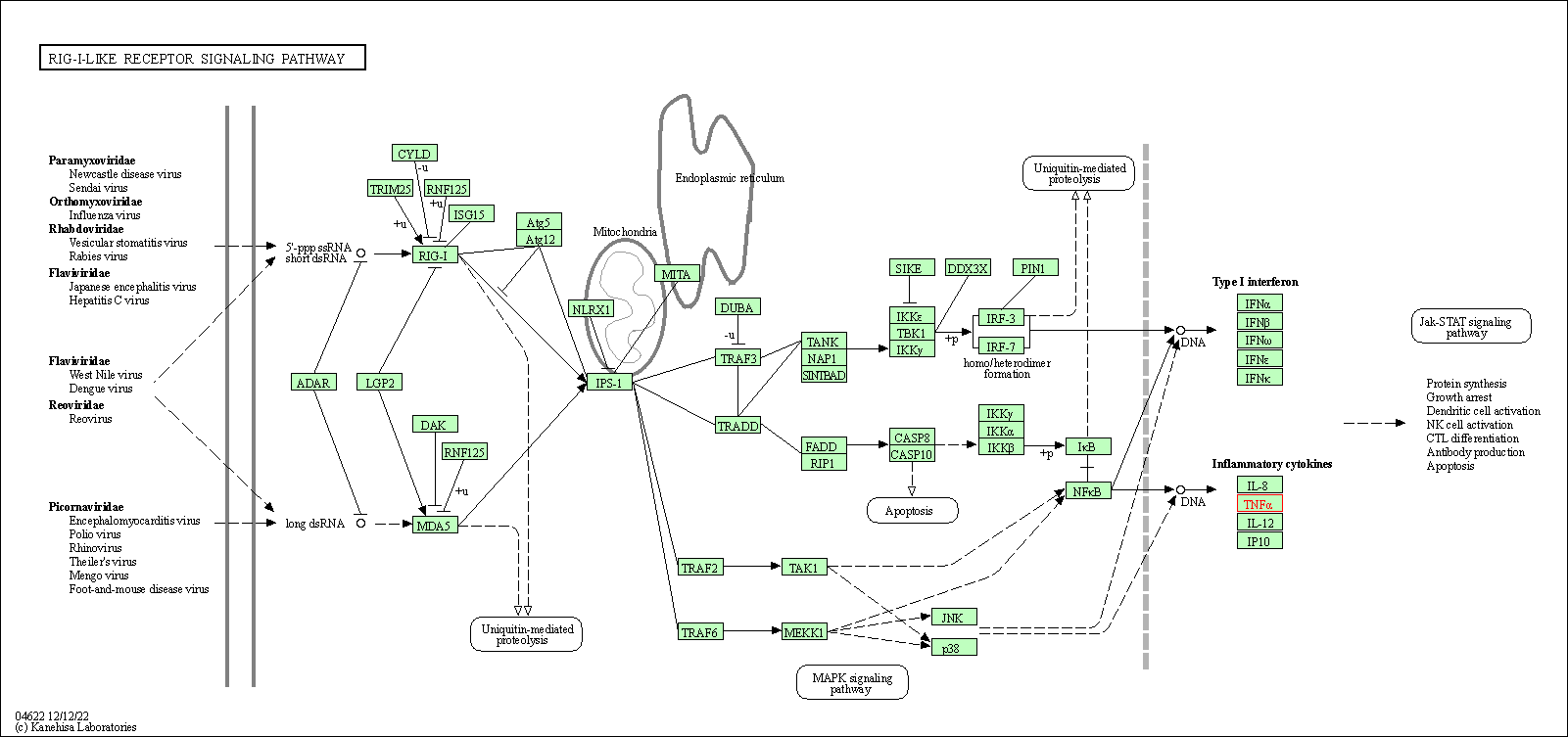
| RIG-I-like receptor signaling pathway | hsa04622 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
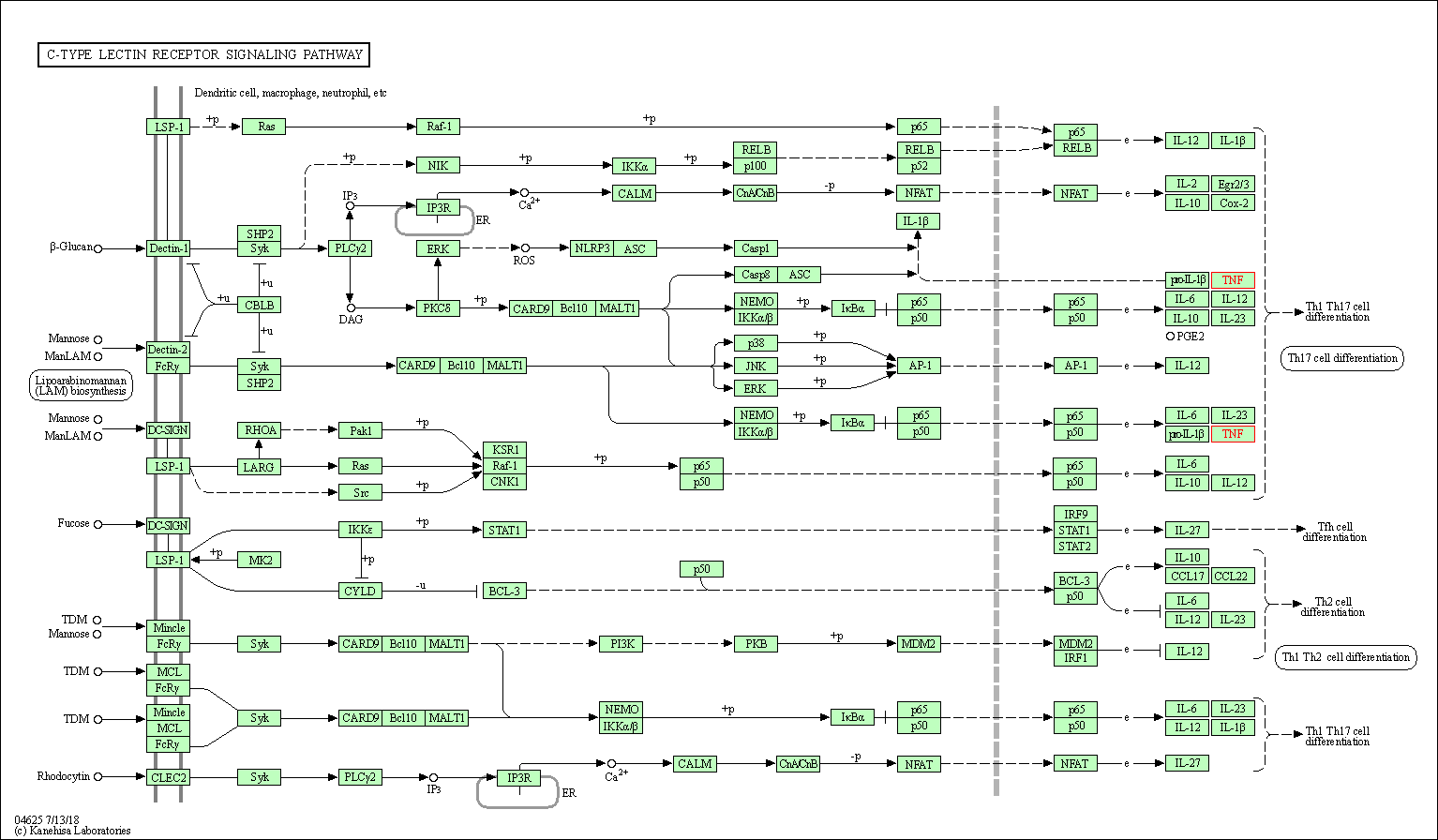
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
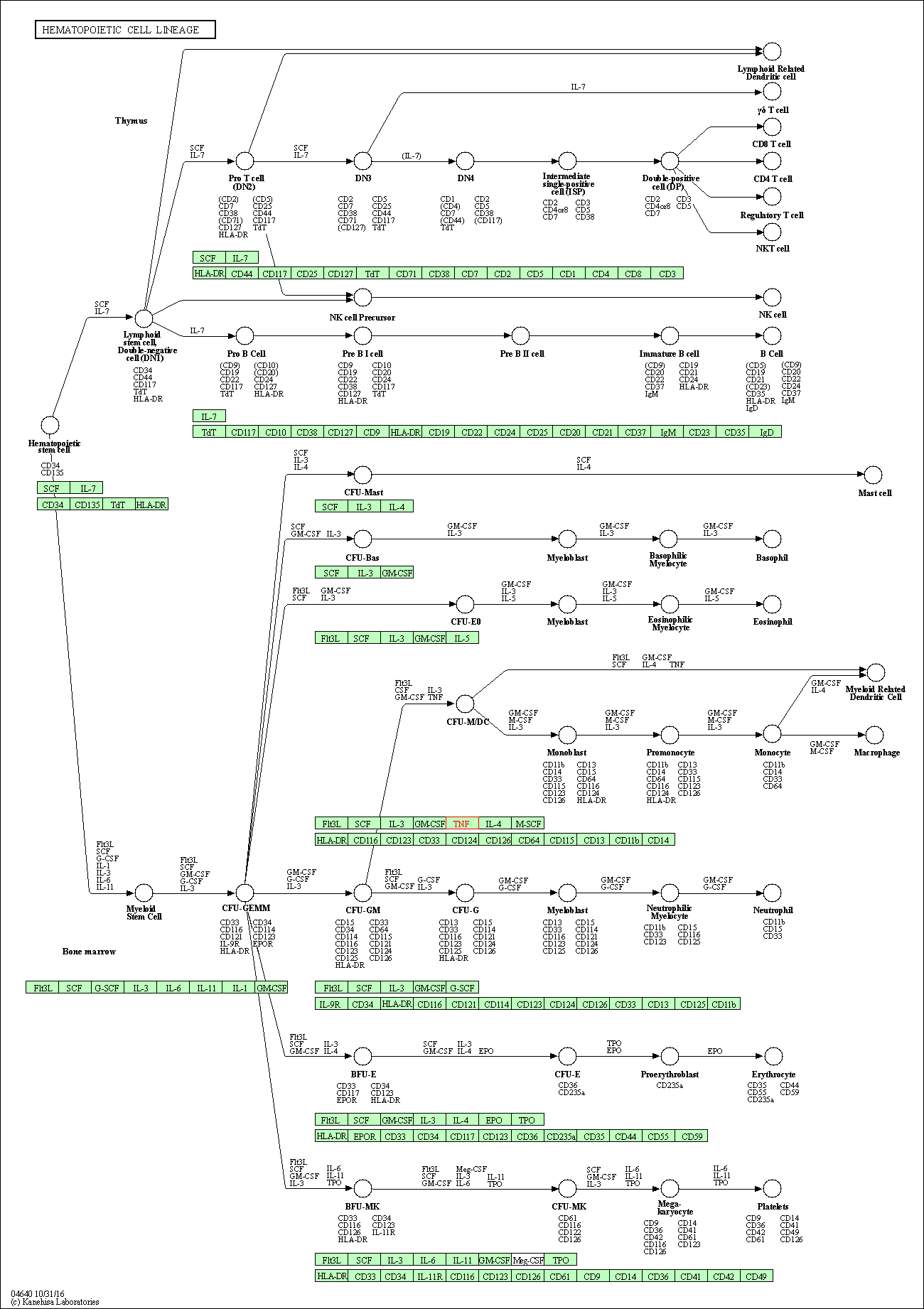
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Natural killer cell mediated cytotoxicity | hsa04650 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| IL-17 signaling pathway | hsa04657 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| T cell receptor signaling pathway | hsa04660 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Fc epsilon RI signaling pathway | hsa04664 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| TNF signaling pathway | hsa04668 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Adipocytokine signaling pathway | hsa04920 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 76 | Degree centrality | 8.17E-03 | Betweenness centrality | 8.97E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.63E-01 | Radiality | 1.45E+01 | Clustering coefficient | 1.60E-01 |
| Neighborhood connectivity | 3.63E+01 | Topological coefficient | 3.75E-02 | Eccentricity | 10 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Adalimumab for psoriasis patients who are non-responders to etanercept: open-label prospective evaluation. J Eur Acad Dermatol Venereol. 2009 Dec;23(12):1394-7. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4860). | |||||
| REF 3 | Molecular targets of rheumatoid arthritis. Inflamm Allergy Drug Targets. 2008 Mar;7(1):53-66. | |||||
| REF 4 | Emerging drugs for rheumatoid arthritis. Expert Opin Emerg Drugs. 2008 Mar;13(1):175-96. | |||||
| REF 5 | Oral pentoxifylline inhibits release of tumor necrosis factor-alpha from human peripheral blood monocytes : a potential treatment for aseptic loose... J Bone Joint Surg Am. 2001 Jul;83(7):1057-61. | |||||
| REF 6 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 7 | ClinicalTrials.gov (NCT02390700) Observational Study of Golimumab Intravenous Infusion | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5004). | |||||
| REF 9 | Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008 Jun;4(6):300-9. | |||||
| REF 10 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2018 | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7331). | |||||
| REF 12 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | |||||
| REF 13 | 2005 approvals: Safety first. Nature Reviews Drug Discovery 5, 92-93 (February 2006). | |||||
| REF 14 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 15 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4262). | |||||
| REF 16 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7095). | |||||
| REF 17 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | |||||
| REF 18 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7327). | |||||
| REF 19 | Thalidomide in multiple myeloma--clinical trials and aspects of drug metabolism and toxicity. Expert Opin Drug Metab Toxicol. 2008 Jul;4(7):973-85. | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800038738) | |||||
| REF 21 | ClinicalTrials.gov (NCT03849404) A Multicenter, Double-blind, Randomized, Parallel-group, Active Control Study to Compare the Efficacy, Safety, and Immunogenicity of AVT02 Versus Humira? in Patients With Moderate-to-Severe Chronic Plaque Psoriasis (ALVOPAD PS). U.S.National Institutes of Health. | |||||
| REF 22 | ClinicalTrials.gov (NCT03398837) Trial to Evaluate Efficacy and Safety of Lenabasum in Diffuse Cutaneous Systemic Sclerosis (RESOLVE-1). U.S. National Institutes of Health. | |||||
| REF 23 | ClinicalTrials.gov (NCT00051467) A Study of TNFerade Biologic With 5-FU and Radiation Therapy for First-Line Treatment of Unresectable Locally Advanced Pancreatic Cancer. U.S. National Institutes of Health. | |||||
| REF 24 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 25 | ClinicalTrials.gov (NCT05068284) A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of ABBV-154 in Subjects With Moderately to Severely Active Crohn's Disease (CD): AIM-CD. U.S.National Institutes of Health. | |||||
| REF 26 | ClinicalTrials.gov (NCT02349451) A Phase 2 Study to Investigate the Safety, Tolerability and Efficacy of ABT-122 in Subjects With Active Psoriatic Arthritis Who Have an Inadequate Response to Methotrexate. U.S. National Institutes of Health. | |||||
| REF 27 | ClinicalTrials.gov (NCT02433340) Phase 2, Multicenter, Open-Label Extension (OLE) Study With ABT-122 in Rheumatoid Arthritis Subjects Who Have Completed the Preceding M12-963 Study | |||||
| REF 28 | Clinical pipeline report, company report or official report of Anacor Pharmaceuticals. | |||||
| REF 29 | ClinicalTrials.gov (NCT02095626) Study in Intensive Care Patients Regarding the Effect of Inhaled AP-301 After Primary Graft Dysfunction After Lung Transplantation. U.S. National Institutes of Health. | |||||
| REF 30 | ClinicalTrials.gov (NCT03830684) A Randomized, Double-blind, Placebo-controlled, Multicenter and Phase IIa Clinical Trial for the Effectiveness and Safety of Baicalein Tablets in the Treatment of Improve Other Aspects of Healthy Adult With Influenza Fever. U.S. National Institutes of Health. | |||||
| REF 31 | ClinicalTrials.gov (NCT01624376) Randomized, Double-blind, Placebo-controlled Study in Patients With Fistulizing Crohn's Disease. U.S. National Institutes of Health. | |||||
| REF 32 | ClinicalTrials.gov (NCT00823173) Exploratory Study on Topical ESBA105 in Acute Anterior Uveitis. U.S. National Institutes of Health. | |||||
| REF 33 | Broad-spectrum modulation of ATP-binding cassette transport proteins by the taxane derivatives ortataxel (IDN-5109, BAY 59-8862) and tRA96023. Cancer Chemother Pharmacol. 2004 May;53(5):363-9. | |||||
| REF 34 | ClinicalTrials.gov (NCT00537667) The SPECTRA Study. U.S. National Institutes of Health. | |||||
| REF 35 | RDP58, a locally active TNF inhibitor, is effective in the dextran sulphate mouse model of chronic colitis. Inflamm Res. 2002 Nov;51(11):522-31. | |||||
| REF 36 | ClinicalTrials.gov (NCT01911234) Clinical Efficacy of TNF-Kinoid in Patients With Rheumatoid Arthritis. U.S. National Institutes of Health. | |||||
| REF 37 | ClinicalTrials.gov (NCT02243787) Safety and Tolerability Study of COVA322 in Patients With Stable Chronic Moderate-to-severe Plaque Psoriasis | |||||
| REF 38 | Emerging drugs for psoriasis. Expert Opin Emerg Drugs. 2009 Mar;14(1):145-63. | |||||
| REF 39 | ClinicalTrials.gov (NCT05890729) A Seamless, Phase 1b/2 Multiple Ascending Dose/Proof of Concept Study of XTMAB-16 in Patients With Pulmonary Sarcoidosis With or Without Extrapulmonary Manifestations. U.S.National Institutes of Health. | |||||
| REF 40 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 41 | ClinicalTrials.gov (NCT01759056) Evaluation of an Oral Anti-TNF Antibody in Patients With Active Ulcerative Colitis. U.S. National Institutes of Health. | |||||
| REF 42 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025369) | |||||
| REF 43 | ClinicalTrials.gov (NCT05533372) Randomized, Double-Blind, Placebo-Controlled, Multiple Ascending Dose Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of IA-14069 in Healthy Subjects, With an Extension to Explore Any Drug-Drug Interaction Potential With Methotrexate (Part 1), and in Patients With Rheumatoid Arthritis, With Preliminary Assessment of Efficacy in Patients (Part 2). U.S.National Institutes of Health. | |||||
| REF 44 | Clinical pipeline report, company report or official report of INmune Bio. | |||||
| REF 45 | ClinicalTrials.gov (NCT01284036) A Single Dose Study Of The Safety And Investigational Product Level Measurement In Healthy Subjects. U.S. National Institutes of Health. | |||||
| REF 46 | ClinicalTrials.gov (NCT01010919) A Study of Chicory for Treatment of Osteoarthritis of the Hip or Knee. U.S. National Institutes of Health. | |||||
| REF 47 | An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022 Nov 16;13:1037983. | |||||
| REF 48 | Clinical pipeline report, company report or official report of Sanofi | |||||
| REF 49 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003884) | |||||
| REF 50 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002771) | |||||
| REF 51 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015375) | |||||
| REF 52 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025635) | |||||
| REF 53 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019215) | |||||
| REF 54 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021664) | |||||
| REF 55 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023971) | |||||
| REF 56 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020354) | |||||
| REF 57 | Emerging disease-modifying therapies for the treatment of motor neuron disease/amyotropic lateral sclerosis. Expert Opin Emerg Drugs. 2007 May;12(2):229-52. | |||||
| REF 58 | Effect of FR133605, a novel cytokine suppressive agent, on bone and cartilage destruction in adjuvant arthritic rats. J Rheumatol. 1996 Oct;23(10):1778-83. | |||||
| REF 59 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007330) | |||||
| REF 60 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800034853) | |||||
| REF 61 | The potential of biologics for the treatment of asthma. Nat Rev Drug Discov. 2012 Dec;11(12):958-72. | |||||
| REF 62 | Etanercept, a TNF antagonist for treatment for psoriatic arthritis and psoriasis. Skin Therapy Lett. 2003 Jan;8(1):1-4. | |||||
| REF 63 | Hughes B: 2009 FDA drug approvals. Nat Rev Drug Discov. 2010 Feb;9(2):89-92. | |||||
| REF 64 | Thalidomide and thalidomide analogues for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2009 Apr 15;(2):CD007351. | |||||
| REF 65 | The thalidomide saga. Int J Biochem Cell Biol. 2007;39(7-8):1489-99. | |||||
| REF 66 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | |||||
| REF 67 | Targeted therapies in myelodysplastic syndromes: ASH 2003 review. Semin Hematol. 2004 Apr;41(2 Suppl 4):13-20. | |||||
| REF 68 | Tumor necrosis factor alpha in the pathogenesis of cerebral malaria. Cell Mol Life Sci. 2003 Aug;60(8):1623-35. | |||||
| REF 69 | Efficacy of different thalidomide regimens for patients with multiple myeloma and its relationship with TNF-alpha level. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008 Dec;16(6):1312-5. | |||||
| REF 70 | Case study: biosimilar anti TNFalpha (Adalimumab) analysis of Fc effector functions. BMC Proc. 2013; 7(Suppl 6): P30. | |||||
| REF 71 | AVT02: An Adalimumab Biosimilar. Clin Drug Investig. 2022 Oct;42(10):875-878. | |||||
| REF 72 | Emerging drugs in neuropathic pain. Expert Opin Emerg Drugs. 2007 Mar;12(1):113-26. | |||||
| REF 73 | TNFerade, an adenovector carrying the transgene for human tumor necrosis factor alpha, for patients with advanced solid tumors: surgical experience... Ann Surg Oncol. 2005 Oct;12(10):825-30. | |||||
| REF 74 | Apoptosis and the FLIP and NF-kappa B proteins as pharmacodynamic criteria for biosimilar TNF-alpha antagonists. Biologics. 2014 Jul 31;8:211-20. | |||||
| REF 75 | Biosimilars in the therapy of inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2014 Jun;26(6):581-7. | |||||
| REF 76 | Discovery of ABBV-154, an anti-TNF Glucocorticoid Receptor Modulator Immunology Antibody-Drug Conjugate (iADC). J Med Chem. 2023 Sep 14;66(17):12544-12558. | |||||
| REF 77 | DOI: 10.1136/annrheumdis-2015-eular.4042 | |||||
| REF 78 | Inhibition of experimental periodontitis by a topical boron-based antimicrobial. J Dent Res. 2008 Feb;87(2):148-52. | |||||
| REF 79 | Identification of a novel boron-containing antibacterial agent (AN0128) with anti-inflammatory activity, for the potential treatment of cutaneous d... Bioorg Med Chem Lett. 2006 Dec 1;16(23):5963-7. | |||||
| REF 80 | Anacor Announces Positive Results From Phase 2 Trial Of Novel Anti-Inflammatory Agent For The Treatment Of Atopic Dermatitis. Anacor Pharmaceuticals. Friday 16 June 2006. | |||||
| REF 81 | AP301, a synthetic peptide mimicking the lectin-like domain of TNF, enhances amiloride-sensitive Na(+) current in primary dog, pig and rat alveolar type II cells. Pulm Pharmacol Ther. 2013 Jun;26(3):356-63. | |||||
| REF 82 | Discovery of the inhibitors of tumor necrosis factor alpha with structure-based virtual screening. Bioorg Med Chem Lett. 2010 Nov 1;20(21):6195-8. | |||||
| REF 83 | Cyclic AMP inhibition of tumor necrosis factor alpha production induced by amyloidogenic C-terminal peptide of Alzheimer's amyloid precursor protein in macrophages: involvement of multiple intracellular pathways and cyclic AMP response element binding protein. Mol Pharmacol. 2003 Mar;63(3):690-8. | |||||
| REF 84 | Pharmacokinetics and posterior segment biodistribution of ESBA105, an anti-TNF-alpha single-chain antibody, upon topical administration to the rabbit eye. Invest Ophthalmol Vis Sci. 2009 Feb;50(2):771-8. | |||||
| REF 85 | New developments in immunosuppressive therapy for heart transplantation. Expert Opin Emerg Drugs. 2009 Mar;14(1):1-21. | |||||
| REF 86 | Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008 May;172(5):1411-8. | |||||
| REF 87 | Modulation of Anti-Tumor Necrosis Factor Alpha (TNF-alpha) Antibody Secretion in Mice Immunized with TNF-alpha Kinoid. Clin Vaccine Immunol. 2012 May; 19(5): 699-703. | |||||
| REF 88 | Bispecific antibodies rise again. Nat Rev Drug Discov. 2014 Nov;13(11):799-801. | |||||
| REF 89 | Clinical pipeline report, company report or official report of Xentria | |||||
| REF 90 | Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-rhTNF Nanomedicine. Clin Cancer Res. 2010 December 15; 16(24): 6139-6149. | |||||
| REF 91 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2023. Adis Insight | |||||
| REF 92 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800033173) | |||||
| REF 93 | US patent application no. 2008,0269,123, Methods for treating polycystic kidney disease (pkd) or other cyst forming diseases. | |||||
| REF 94 | An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022 Nov 16;13:1037983. | |||||
| REF 95 | CDP571, a humanized monoclonal antibody to tumour necrosis factor-alpha, for steroid-dependent Crohn's disease: a randomized, double-blind, placebo... Aliment Pharmacol Ther. 2006 Mar 1;23(5):617-28. | |||||
| REF 96 | Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit Care Med. 2001 Apr;29(4):765-9. | |||||
| REF 97 | AGIX-4207 [2-[4-[[1-[[3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl]thio]-1-methylethyl]thio]-2,6-bis(1,1-dimethylethyl)phenoxy]acetic acid], a novel ... J Pharmacol Exp Ther. 2005 May;313(2):492-501. | |||||
| REF 98 | CombinatoRx Drug Candidate CRx-191 Demonstrates Positive Phase 2 Results In Psoriasis. CombinatoRx. 2008. | |||||
| REF 99 | IND Filed for AME-527, Monoclonal Antibody That Is a Potential Treatment for Rheumatoid Arthritis. P&T Community. 2015. | |||||
| REF 100 | Therapeutic Vaccination with TNF-Kinoid in TNF Antagonist-Resistant Rheumatoid Arthritis: A Phase II Randomized, Controlled Clinical Trial. PLoS One. 2014; 9(12): e113465. | |||||
| REF 101 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 102 | US patent application no. 8007,790, Methods for treating polycystic kidney disease (pkd) or other cyst forming diseases. | |||||
| REF 103 | Effect of the carbocyclic nucleoside analogue MDL 201,112 on inhibition of interferon-gamma-induced priming of Lewis (LEW/N) rat macrophages for enhanced respiratory burst and MHC class II Ia+ antigen expression. J Leukoc Biol. 1994 Aug;56(2):133-44. | |||||
| REF 104 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 105 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||||
| REF 106 | Discovery of gamma-lactam hydroxamic acids as selective inhibitors of tumor necrosis factor alpha converting enzyme: design, synthesis, and structu... J Med Chem. 2002 Nov 7;45(23):4954-7. | |||||
| REF 107 | Beta-aryl-succinic acid hydroxamates as dual inhibitors of matrix metalloproteinases and tumor necrosis factor alpha converting enzyme. J Med Chem. 2002 May 23;45(11):2289-93. | |||||
| REF 108 | A novel anti-rheumatic drug suppresses tumor necrosis factor-alpha and augments interleukin-10 in adjuvant arthritic rats. Eur J Pharmacol. 2000 Dec 15;409(3):331-5. | |||||
| REF 109 | Structural Basis of Small-Molecule Aggregate Induced Inhibition of a Protein-Protein Interaction. J Med Chem. 2017 Apr 27;60(8):3511-3517. | |||||
| REF 110 | Natural Conformational Sampling of Human TNFAlpha Visualized by Double Electron-Electron Resonance. Biophys J. 2017 Jul 25;113(2):371-380. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

