Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DW4O2J
|
|||
| Drug Name |
J147
|
|||
| Synonyms |
j147; J-147; 1146963-51-0; UNII-Z41H3C5BT9; 1807913-16-1; Z41H3C5BT9; N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N-[(E)-(3-methoxyphenyl)methylideneamino]acetamide; N-(2,4-Dimethylphenyl)-2,2,2-trifluoro-N'-(3-methoxybenzylidene)acetohydrazide; Acetic acid, 2,2,2-trifluoro-, 1-(2,4-dimethylphenyl)-2-((3-methoxyphenyl)methylene)hydrazide; N-(2,4-Dimethylphenyl)-2,2,2-trifluoro-N'-[(E)-(3-methoxyphenyl)methylene]acetohydrazide; N-(2,4-Dimethylphenyl)-2,2,2-trifluoro-N-((E)-(3-methoxyphenyl)methylideneamino)acetamide; Acetic acid, 2,2,2-trifluoro-, (2E)-1-(2,4-dimethylphenyl)-2-((3-methoxyphenyl)methylene)hydrazide; Acetic acid, 2,2,2-trifluoro-, 1-(2,4-dimethylphenyl)-2-[(3-methoxyphenyl)methylene]hydrazide; N-(2,4-DIMETHYLPHENYL)-2,2,2-TRIFLUORO-N'-[(1E)-(3-METHOXYPHENYL)METHYLIDENE]ACETOHYDRAZIDE; CHEMBL2387144; SCHEMBL12995834; SCHEMBL21294999; 2,2,2-Trifluoroacetic acid 1-(2,4-Dimethylphenyl)-2-[(3-methoxyphenyl)methylene]hydrazide; CHEBI:192601; HYMZAYGFKNNHDN-SSDVNMTOSA-N; DTXSID501045787; HMS3886O17; EX-A2235; MFCD25976644; s5269; AKOS024458485; CCG-268030; CS-3688; DB13957; AC-35232; AS-16718; HY-13779; A894156; (E)-N-(2,4-Dimethylphenyl)-2,2,2-trifluoro-N'-(3-methoxybenzylidene)acetohydrazide; N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N'-[(3-methoxyphenyl)methylidene]acetohydrazide; N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N'-[(E)-(3-methoxyphenyl)methylidene]acetohydrazide; N-(2,4-dimethylphenyl)-2,2,2-triluoro-N-[(E)-(3-methoxyphenyl)methylideneamino]acetamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 1 | [1] | |
| Company |
Abrexa
|
|||
| Structure |
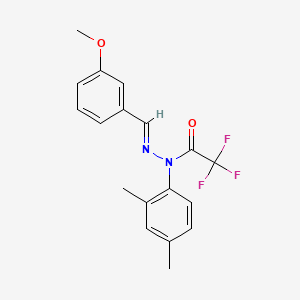 |
Download2D MOL |
||
| Formula |
C18H17F3N2O2
|
|||
| Canonical SMILES |
CC1=CC(=C(C=C1)N(C(=O)C(F)(F)F)N=CC2=CC(=CC=C2)OC)C
|
|||
| InChI |
InChI=1S/C18H17F3N2O2/c1-12-7-8-16(13(2)9-12)23(17(24)18(19,20)21)22-11-14-5-4-6-15(10-14)25-3/h4-11H,1-3H3/b22-11+
|
|||
| InChIKey |
HYMZAYGFKNNHDN-SSDVNMTOSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | ATP synthase (ATPS) | Target Info | inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03838185) A Phase I, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety, Tolerability and Pharmacokinetics of Single Ascending Oral Doses of J147 in Healthy Young Volunteers and Healthy Elderly Volunteers. U.S.National Institutes of Health. | |||
| REF 2 | Broadening the horizon: Integrative pharmacophore-based and cheminformatics screening of novel chemical modulators of mitochondria ATP synthase towards interventive Alzheimer's disease therapy. Med Hypotheses. 2019 Sep;130:109277. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

