Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DTG28Y
|
|||
| Drug Name |
LY3473329
|
|||
| Synonyms |
Muvalaplin; Muvalaplin [USAN]; HSU2KY4EFK; LY3473329; UNII-HSU2KY4EFK; WHO 12607; 2565656-70-2; LY-3473329; (2S,2'S,2''S)-3,3',3''-((nitrilotris(methylene))tris(benzene-3,1-diyl))tris(2-((R)-pyrrolidin-3-yl)propanoic acid); 3-Pyrrolidineacetic acid, alpha,alpha',alpha''- (nitrilotris(methylene-3,1-phenylenemethylene))tris-,(alphaS,alpha'S,alpha''S,3R,3'R,3''R)-; muvalaplin [INN]; CHEMBL4802585; SCHEMBL22750411; GTPL12939; HY-152857; CS-0641200; (2S)-3-[3-[[bis[[3-[(2S)-2-carboxy-2-[(3R)-pyrrolidin-3-yl]ethyl]phenyl]methyl]amino]methyl]phenyl]-2-[(3R)-pyrrolidin-3-yl]propanoic acid; (2S)-3-[3-[[bis[[3-[(2S)-2-carboxy-2-[(3R)-pyrrolidin-3-yl]ethyl]phenyl]methyl]amino]phenyl]-2-[(3R)-pyrrolidin-3-yl]propanoic acid; 3-Pyrrolidineacetic acid, alpha,alpha',alpha''- [nitrilotris(methylene-3,1-phenylenemethylene)]tris-, (alphaS,alpha'S,alpha''S,3R,3'R,3''R)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Cardiovascular disease [ICD-11: BA00-BE2Z] | Phase 2 | [1] | |
| Company |
Lilly
|
|||
| Structure |
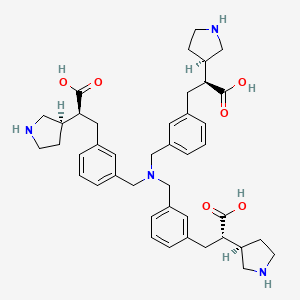 |
Download2D MOL
|
||
| Formula |
C42H54N4O6
|
|||
| Canonical SMILES |
C1CNCC1C(CC2=CC(=CC=C2)CN(CC3=CC=CC(=C3)CC(C4CCNC4)C(=O)O)CC5=CC=CC(=C5)CC(C6CCNC6)C(=O)O)C(=O)O
|
|||
| InChI |
InChI=1S/C42H54N4O6/c47-40(48)37(34-10-13-43-22-34)19-28-4-1-7-31(16-28)25-46(26-32-8-2-5-29(17-32)20-38(41(49)50)35-11-14-44-23-35)27-33-9-3-6-30(18-33)21-39(42(51)52)36-12-15-45-24-36/h1-9,16-18,34-39,43-45H,10-15,19-27H2,(H,47,48)(H,49,50)(H,51,52)/t34-,35-,36-,37-,38-,39-/m0/s1
|
|||
| InChIKey |
BRLGERLDHZRETI-BGBFCPIGSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Apolipoprotein(a) (LPA) | Target Info | Inhibitor | [2] |
| NetPath Pathway | IL2 Signaling Pathway | |||
| Panther Pathway | Plasminogen activating cascade | |||
| Pathway Interaction Database | amb2 Integrin signaling | |||
| Reactome | LDL-mediated lipid transport | |||
| WikiPathways | Lipid digestion, mobilization, and transport | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05563246) KRAKEN: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of Oral Once-Daily LY3473329 in Adults With Elevated Lipoprotein(a) at High Risk for Cardiovascular Events. U.S.National Institutes of Health. | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2023. Adis Insight | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

