Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D37IQM
|
|||
| Drug Name |
JDQ443
|
|||
| Synonyms |
JDQ-443; Opnurasib; JDQ443; 2653994-08-0; NVP-JDQ443; NVP-JDQ-443; JDQ 443; (S)-JDQ-443; 1-[6-[4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methylindazol-5-yl)pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl]prop-2-en-1-one; opnurasib [INN]; Q3W0H3V1LQ; CHEMBL5077861; JDQ 443 [WHO-DD]; SCHEMBL23533580; GTPL11715; GLXC-25533; EX-A5693; BDBM50579985; NSC846146; AKOS040757949; AT36708; compound 5 [PMID: 35404998]; HY-139612A; NSC-846146; example 1a [WO2021120890A1]; MS-29737; CS-0226220; CS-0311034; -PROPEN-1-ONE, 1-(6-((4R)-4-(5-CHLORO-6-METHYL-1H-INDAZOL-4-YL)-5-METHYL-3-(1-METHYL-1H-INDAZOL-5-YL)-1H-PYRAZOL-1-YL)-2-AZASPIRO(3.3)HEPT-2-YL)-; 1-(6-((4S)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl)-2-azaspiro[3.3]heptan-2-yl)prop-2-en-1-one; 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-inda zol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]- 2-Propen-1-one; 1-[6-[(4R)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-2-propen-1-one; 1-{6-[(4M)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5- methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2- azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 1-{6-[(4M)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 2-Propen-1-one, 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-; 2653994-10-4
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Non-small-cell lung cancer [ICD-11: 2C25] | Phase 3 | [1] | |
| Company |
Novartis
|
|||
| Structure |
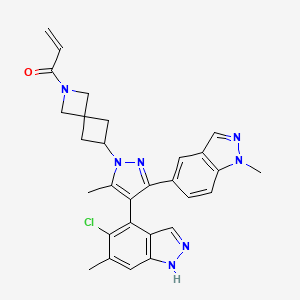 |
Download2D MOL |
||
| Formula |
C29H28ClN7O
|
|||
| Canonical SMILES |
CC1=CC2=C(C=NN2)C(=C1Cl)C3=C(N(N=C3C4=CC5=C(C=C4)N(N=C5)C)C6CC7(C6)CN(C7)C(=O)C=C)C
|
|||
| InChI |
InChI=1S/C29H28ClN7O/c1-5-24(38)36-14-29(15-36)10-20(11-29)37-17(3)25(26-21-13-31-33-22(21)8-16(2)27(26)30)28(34-37)18-6-7-23-19(9-18)12-32-35(23)4/h5-9,12-13,20H,1,10-11,14-15H2,2-4H3,(H,31,33)
|
|||
| InChIKey |
AZUYLZMQTIKGSC-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | KRAS G12C mutant (KRAS G12C) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05132075) A Randomized, Controlled, Open Label, Phase III Study Evaluating the Efficacy and Safety of JDQ443 Versus Docetaxel in Previously Treated Subjects With Locally Advanced or Metastatic KRAS G12C Mutant Non-small Cell Lung Cancer. U.S.National Institutes of Health. | |||
| REF 2 | Discovery, Preclinical Characterization, and Early Clinical Activity of JDQ443, a Structurally Novel, Potent, and Selective Covalent Oral Inhibitor of KRASG12C. Cancer Discov. 2022 Jun 2;12(6):1500-1517. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

