Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Y6ZK
|
|||
| Former ID |
DNC000764
|
|||
| Drug Name |
IB-MECA
|
|||
| Synonyms |
IB-Meca; 152918-18-8; piclidenoson; CF-101; 3-IB-Meca; N(6)-Ibamu; CF 101; Cf101; N(6)-(3-iodobenzyl)-5'-N-methylcarboxamidoadenosine; UNII-30679UMI0N; N(6)-(3-Iodobenzyl)adenosine-5'-N-methyluronamide; 1-Deoxy-1-(6-(((3-iodophenyl)methyl)amino)-9H-purin-9-yl)-N-methyl-beta-D-ribofuranuronamide; CHEMBL119709; CHEBI:73286; 30679UMI0N; RPR-113090; 3-iodobenzyl-5'-N-methylcarboxamidoadenosine; N(6)-(3-iodo-benzyl)adenosine-5'-N-methyluronamide; 3-IB-MECA
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Psoriasis vulgaris [ICD-11: EA90; ICD-9: 696] | Phase 3 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 3 | [2], [3], [4] | ||
| Plaque psoriasis [ICD-11: EA90.0; ICD-10: L40.0] | Phase 2/3 | [5] | ||
| Rheumatoid arthritis [ICD-11: FA20] | Phase 2 | [1] | ||
| Structure |
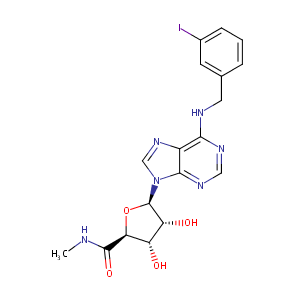 |
Download2D MOL |
||
| Formula |
C18H19IN6O4
|
|||
| Canonical SMILES |
CNC(=O)C1C(C(C(O1)N2C=NC3=C(N=CN=C32)NCC4=CC(=CC=C4)I)O)O
|
|||
| InChI |
1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1
|
|||
| InChIKey |
HUJXGQILHAUCCV-MOROJQBDSA-N
|
|||
| CAS Number |
CAS 152918-18-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7978518, 10240823, 12014966, 14884738, 14909248, 24277717, 26752055, 26752056, 29303901, 50068252, 50104709, 53789321, 56463617, 57340522, 75764681, 90341246, 91704557, 103344811, 104012075, 104419080, 124800870, 126671545, 128114796, 134340105, 135080042, 135650362, 135653740, 135698154, 140688645, 162169281, 162226817, 163123389, 171579213, 175427023, 188627623, 198971672, 204371128, 223660144, 226783446, 241182098, 241375196, 250124320, 252215560, 252432477, 252450996
|
|||
| ChEBI ID |
CHEBI:73286
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adenosine A3 receptor (ADORA3) | Target Info | Agonist | [6], [7] |
| Reactome | Adenosine P1 receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | Nucleotide GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 422). | |||
| REF 3 | Methotrexate enhances the anti-inflammatory effect of CF101 via up-regulation of the A3 adenosine receptor expression. Arthritis Res Ther. 2006;8(6):R169. | |||
| REF 4 | CF101, an agonist to the A3 adenosine receptor, enhances the chemotherapeutic effect of 5-fluorouracil in a colon carcinoma murine model. Neoplasia. 2005 Jan;7(1):85-90. | |||
| REF 5 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 6 | A role for central A3-adenosine receptors. Mediation of behavioral depressant effects. FEBS Lett. 1993 Dec 20;336(1):57-60. | |||
| REF 7 | A3 adenosine receptor as a target for cancer therapy. Anticancer Drugs. 2002 Jun;13(5):437-43. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

