Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0TY8X
|
|||
| Former ID |
DNC005902
|
|||
| Drug Name |
4-(2-aminopyrimidin-4-ylamino)benzenesulfonamide
|
|||
| Synonyms |
NSC135784; 2153-13-1; 4-[(2-aminopyrimidin-4-yl)amino]benzenesulfonamide; UNII-1DW8U29X8S; CHEMBL6633; 1DW8U29X8S; 4-((2-amino-4-pyrimidinyl)amino)benzenesulfonamide; NSC-135784; NSC683526; AC1L1KSM; NCIStruc2_000899; NCIStruc1_000852; Oprea1_770349; SCHEMBL3791868; ZINC56465; CTK4E7061; BDBM10873; NCI135784; NCGC00014345; CCG-38281; AKOS030546962; aromatic/heteroaromatic sulfonamide 18; MCULE-1716829198; NCGC00097454-01; NCGC00014345-02; NCI60_000801; EN300-24853; 4-(2-Amino-4-pyrimidinylamino)benzenesulfonamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
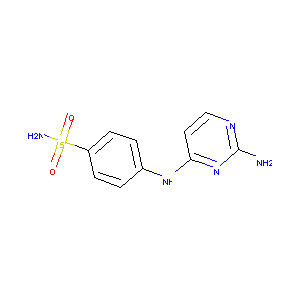 |
Download2D MOL |
||
| Formula |
C10H11N5O2S
|
|||
| Canonical SMILES |
C1=CC(=CC=C1NC2=NC(=NC=C2)N)S(=O)(=O)N
|
|||
| InChI |
1S/C10H11N5O2S/c11-10-13-6-5-9(15-10)14-7-1-3-8(4-2-7)18(12,16)17/h1-6H,(H2,12,16,17)(H3,11,13,14,15)
|
|||
| InChIKey |
QOAXQANKSMHFKJ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 2153-13-1
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Cloning, expression, post-translational modifications and inhibition studies on the latest mammalian carbonic anhydrase isoform, CA XV. J Med Chem. 2009 Feb 12;52(3):646-54. | |||
| REF 2 | Carbonic anhydrase inhibitors. Inhibition studies of a coral secretory isoform by sulfonamides. Bioorg Med Chem. 2009 Jul 15;17(14):5054-8. | |||
| REF 3 | Carbonic anhydrase inhibitors. Characterization and inhibition studies of the most active beta-carbonic anhydrase from Mycobacterium tuberculosis, ... Bioorg Med Chem Lett. 2009 Dec 1;19(23):6649-54. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

