Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0R9US
|
|||
| Former ID |
DIB012665
|
|||
| Drug Name |
GSK2140944
|
|||
| Synonyms |
Gepotidacin
Click to Show/Hide
|
|||
| Indication | Neisseria gonorrhoeae infection [ICD-11: 1A7Z; ICD-10: A54, A54.9] | Phase 3 | [1] | |
| Urinary tract infection [ICD-11: GC08; ICD-10: N39, N39.0] | Phase 3 | [2] | ||
| Acute bacterial skin infection [ICD-11: 1C41] | Phase 2 | [3] | ||
| Company |
Glaxosmithkline
|
|||
| Structure |
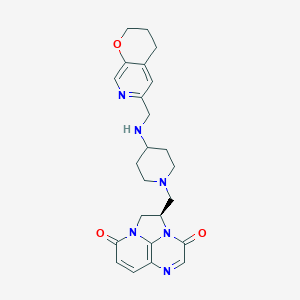 |
Download2D MOL |
||
| Formula |
C24H28N6O3
|
|||
| Canonical SMILES |
C1CC2=CC(=NC=C2OC1)CNC3CCN(CC3)CC4CN5C(=O)C=CC6=C5N4C(=O)C=N6
|
|||
| InChI |
1S/C24H28N6O3/c31-22-4-3-20-24-29(22)15-19(30(24)23(32)13-27-20)14-28-7-5-17(6-8-28)25-11-18-10-16-2-1-9-33-21(16)12-26-18/h3-4,10,12-13,17,19,25H,1-2,5-9,11,14-15H2/t19-/m1/s1
|
|||
| InChIKey |
PZFAZQUREQIODZ-LJQANCHMSA-N
|
|||
| CAS Number |
CAS 1075236-89-3
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Target Info | Inhibitor | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04010539) A Study Evaluating Efficacy and Safety of Gepotidacin Compared With Ceftriaxone Plus Azithromycin in the Treatment of Uncomplicated Urogenital Gonorrhea. U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT04020341) A Study to Evaluate Efficacy and Safety of Gepotidacin in the Treatment of Uncomplicated Urinary Tract Infection (UTI). U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Determination of disk diffusion and MIC quality control guidelines for GSK2140944, a novel bacterial type II topoisomerase inhibitor antimicrobial agent. J Clin Microbiol. 2014 Jul;52(7):2629-32. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

