Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0R0UJ
|
|||
| Former ID |
DAP000580
|
|||
| Drug Name |
Pargyline
|
|||
| Synonyms |
Benzylmethylpropargylamine; Benzylmethylpropynylamine; Eudatin; Eutron; Methylbenzylpropynylamine; Paragyline; Pargilina; Pargylamine; Pargylin; Pargylinum; Supirdyl; A 19120; MO 911; Pargilina [INN-Spanish]; Pargyline (INN); Pargyline [INN:BAN]; Pargylinum [INN-Latin]; Benzyl-methyl-2-propinylamin; Benzyl-methyl-2-propinylamin [Czech]; N-Methyl-N-benzylpropynylamine; N-Methyl-N-propargylbenzylamine; N-Methyl-N-2-propynylbenzylamine; N-Benzyl-N-methyl-2-propynylamine; N-Benzyl-N-methyl-2-propyn-1; N-Benzyl-N-methyl-2-propyn-1-amine; N-benzyl-N-methylprop-2-yn-1-amine; N-methyl-N-(phenylmethyl)prop-2-yn-1-amine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypertension [ICD-11: BA00-BA04] | Approved | [1] | |
| Muscular dystrophy [ICD-11: 8C70; ICD-10: G71.0] | Patented | [2] | ||
| Skin imperfections [ICD-11: EK71; ICD-10: L91.8] | Patented | [2] | ||
| Therapeutic Class |
Antihypertensive Agents
|
|||
| Company |
Abbott Laboratories Pharmaceutical Products Div
|
|||
| Structure |
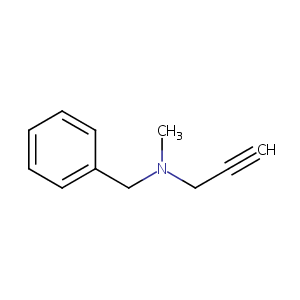 |
Download2D MOL |
||
| Formula |
C11H13N
|
|||
| Canonical SMILES |
CN(CC#C)CC1=CC=CC=C1
|
|||
| InChI |
1S/C11H13N/c1-3-9-12(2)10-11-7-5-4-6-8-11/h1,4-8H,9-10H2,2H3
|
|||
| InChIKey |
DPWPWRLQFGFJFI-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 555-57-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9618, 794485, 3133951, 4905557, 8152879, 10527079, 11111668, 11111669, 11335228, 11360467, 11363535, 11366097, 11368659, 11371759, 11374057, 11376821, 11461439, 11466211, 11467331, 11484993, 11485762, 11488855, 11490356, 11492284, 11494455, 15171008, 24897164, 29223774, 46507368, 47216615, 47290968, 47736296, 47810590, 48034936, 48034937, 48034938, 48034939, 48334307, 48416382, 49698875, 49893341, 50100328, 50104247, 50735625, 56369647, 57322398, 85177273, 85787887, 85856309, 90340843
|
|||
| ChEBI ID |
CHEBI:7930
|
|||
| SuperDrug ATC ID |
C02KC01
|
|||
| SuperDrug CAS ID |
cas=000555577
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Monoamine oxidase (MAO) | Target Info | Inhibitor | [2] |
| Monoamine oxidase type B (MAO-B) | Target Info | Inhibitor | [3] | |
| BioCyc | Superpathway of tryptophan utilization | |||
| Tryptophan degradation via tryptamine | ||||
| Dopamine degradation | ||||
| Putrescine degradation III | ||||
| Noradrenaline and adrenaline degradation | ||||
| KEGG Pathway | Glycine, serine and threonine metabolism | |||
| Arginine and proline metabolism | ||||
| Histidine metabolism | ||||
| Tyrosine metabolism | ||||
| Phenylalanine metabolism | ||||
| Tryptophan metabolism | ||||
| Drug metabolism - cytochrome P450 | ||||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Dopaminergic synapse | ||||
| Cocaine addiction | ||||
| Amphetamine addiction | ||||
| Alcoholism | ||||
| Panther Pathway | Adrenaline and noradrenaline biosynthesis | |||
| 5-Hydroxytryptamine degredation | ||||
| Dopamine receptor mediated signaling pathway | ||||
| Pathway Interaction Database | Alpha-synuclein signaling | |||
| WikiPathways | Tryptophan metabolism | |||
| Dopamine metabolism | ||||
| Phase 1 - Functionalization of compounds | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Novel monoamine oxidase inhibitors: a patent review (2012 - 2014).Expert Opin Ther Pat. 2015 Jan;25(1):91-110. | |||
| REF 3 | Dose-dependent activation of distinct hypertrophic pathways by serotonin in cardiac cells. Am J Physiol Heart Circ Physiol. 2009 Aug;297(2):H821-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

