Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0L1YM
|
|||
| Former ID |
DPR000148
|
|||
| Drug Name |
McN3377
|
|||
| Synonyms |
Fenobamum; Fenobam (TN); Fenobamum [INN-Latin]; McN-3377; NPL-2009; N-(3-Chlorophenyl)-N'-(4,5-dihydro-1-methyl-4-oxo-1H-imidazol-2-yl)urea; N-(3-Chlorophenyl)-N'-(4,5-dihydro-1-methyl-4-oxo-1H-imidazole-2-yl)urea; 1-(3-chlorophenyl)-3-(3-methyl-5-oxo-4H-imidazol-2-yl)urea
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Fragile X syndrome [ICD-11: LD55; ICD-10: Q99.2] | Phase 1/2 | [1], [2] | |
| Therapeutic Class |
Psychiatric
|
|||
| Company |
McNeil Laboratories
|
|||
| Structure |
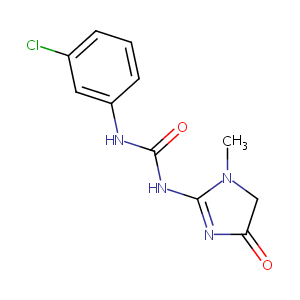 |
Download2D MOL |
||
| Canonical SMILES |
CN1CC(=O)N=C1NC(=O)NC2=CC(=CC=C2)Cl
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7698550, 10255018, 12015575, 15368151, 24894721, 26753296, 46237428, 50076506, 57350010, 79662796, 85209784, 85788195, 90341711, 90438823, 103088281, 103088282, 103156738, 103156739, 103546087, 104081432, 113450413, 117595975, 121324203, 121361629, 134221954, 135116692, 135650255, 135651153, 135697547, 136309293, 137133037, 144206177, 144207065, 162023388, 162221725, 162887753, 163134645, 163564796, 164175140, 164814496, 167250227, 170466272, 174006275, 175612574, 177513142, 179150698, 184546104, 198946610, 216088083, 223704488
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1434). | |||
| REF 2 | ClinicalTrials.gov (NCT00637221) Open Label Study Investigating Safety and Efficacy of NPL2009 50 mg - 150 mg on Prepulse Inhibition Tests and Continuous Performance Tasks, Adults With Fragile X Syndrome. U.S. National Institutes of Health. | |||
| REF 3 | Glutamate- and GABA-based CNS therapeutics. Curr Opin Pharmacol. 2006 Feb;6(1):7-17. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

