Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0H6QU
|
|||
| Former ID |
DIB015641
|
|||
| Drug Name |
Apomorphine
|
|||
| Synonyms |
Apomorphine (intranasal, Parkinson's disease)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Parkinson disease [ICD-11: 8A00.0; ICD-9: 332] | Approved | [1], [2] | |
| Idiopathic parkinson disease [ICD-11: 8A00.0Z; ICD-9: 332] | Phase 3 | [3] | ||
| Sexual dysfunction [ICD-11: HA00-HA01; ICD-9: 302.7] | Phase 2 | [4] | ||
| Company |
Archimedes Pharma Ltd
|
|||
| Structure |
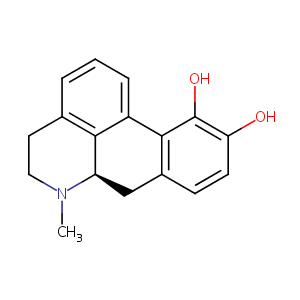 |
Download2D MOL |
||
| Formula |
C17H17NO2
|
|||
| Canonical SMILES |
CN1CCC2=C3C1CC4=C(C3=CC=C2)C(=C(C=C4)O)O
|
|||
| InChI |
1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1
|
|||
| InChIKey |
VMWNQDUVQKEIOC-CYBMUJFWSA-N
|
|||
| CAS Number |
CAS 58-00-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7978719, 8153735, 11114270, 11466129, 11467249, 11485757, 14823961, 14848260, 24262970, 26752261, 29225019, 46508653, 47216673, 47365074, 47365075, 47662161, 47662162, 47662163, 48110346, 48184887, 48259117, 48415574, 49658769, 49698343, 49871434, 50322702, 51091791, 57323108, 85788349, 85856298, 92308726, 92729815, 93622853, 103167211, 103916308, 104310845, 124887059, 124887060, 126522630, 129497604, 134337595, 134971357, 135304977, 135649950, 137001467, 142333205, 144204518, 160964058, 163667986, 164788060
|
|||
| ChEBI ID |
CHEBI:48538
|
|||
| ADReCS Drug ID | BADD_D00152 ; BADD_D00153 | |||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Parabacteroides distasonis
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Parabacteroides distasonis was decreased by R(-) Apomorphine hydrochloride hemihydrate (adjusted p-values: 1.64E-04). | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 33). | |||
| REF 2 | ClinicalTrials.gov (NCT02339064) Infusion of Apomorphine: Long-term Safety Study. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Intranasal apomorphine. Nastech Pharmaceutical. IDrugs. 2004 May;7(5):483-8. | |||
| REF 5 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 6 | Dopamine D(2/3) receptor occupancy of apomorphine in the nonhuman primate brain--a comparative PET study with [11C]raclopride and [11C]MNPA. Synapse. 2009 May;63(5):378-89. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

