Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E6BB
|
|||
| Former ID |
DNCL003635
|
|||
| Drug Name |
Plazomicin
|
|||
| Synonyms |
ACHN-490; UNII-LYO9XZ250J; 1154757-24-0; LYO9XZ250J; Plazomicin [USAN:INN]; Plazomicin (USAN); ZINC68150640; DB12615; D10151; D-Streptamine,
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Bronchitis [ICD-11: CA20; ICD-10: J40] | Approved | [1] | |
| Prostate disease [ICD-11: GA91] | Approved | [1] | ||
| Urinary tract infection [ICD-11: GC08; ICD-10: N39, N39.0] | Approved | [1] | ||
| Pancreatic cancer [ICD-11: 2C10] | Phase 3 | [2] | ||
| Company |
Achaogen
|
|||
| Structure |
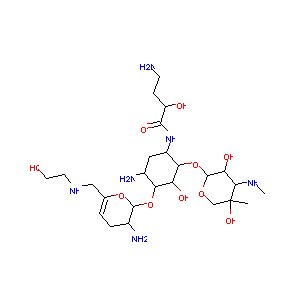 |
Download2D MOL |
||
| Formula |
C25H48N6O10
|
|||
| Canonical SMILES |
CC1(COC(C(C1NC)O)OC2C(CC(C(C2O)OC3C(CC=C(O3)CNCCO)N)N)NC(=O)C(CCN)O)O
|
|||
| InChI |
1S/C25H48N6O10/c1-25(37)11-38-24(18(35)21(25)29-2)41-20-15(31-22(36)16(33)5-6-26)9-14(28)19(17(20)34)40-23-13(27)4-3-12(39-23)10-30-7-8-32/h3,13-21,23-24,29-30,32-35,37H,4-11,26-28H2,1-2H3,(H,31,36)/t13-,14+,15-,16+,17+,18-,19-,20+,21-,23-,24-,25+/m1/s1
|
|||
| InChIKey |
IYDYFVUFSPQPPV-PEXOCOHZSA-N
|
|||
| CAS Number |
CAS 1154757-24-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Mitochondrial rRNA methyltransferase 2 (MRM2) | Target Info | Inhibitor | [1] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | In vitro activity of plazomicin against -lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE).J Antimicrob Chemother. 2017 Oct 1;72(10):2792-2795. | |||
| REF 2 | ClinicalTrials.gov (NCT01970371) A Study of Plazomicin Compared With Colistin in Patients With Infection Due to Carbapenem-Resistant Enterobacteriaceae (CRE). U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

